FMRP phosphorylation reveals an immediate-early signaling pathway triggered by group I mGluR and mediated by PP2A
- PMID: 18160642
- PMCID: PMC6673448
- DOI: 10.1523/JNEUROSCI.2969-07.2007
FMRP phosphorylation reveals an immediate-early signaling pathway triggered by group I mGluR and mediated by PP2A
Abstract
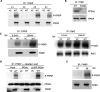
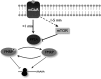
Fragile X syndrome is a common form of inherited mental retardation and is caused by loss of fragile X mental retardation protein (FMRP), a selective RNA-binding protein that influences the translation of target messages. Here, we identify protein phosphatase 2A (PP2A) as an FMRP phosphatase and report rapid FMRP dephosphorylation after immediate group I metabotropic glutamate receptor (mGluR) stimulation (<1 min) in neurons caused by enhanced PP2A enzymatic activity. In contrast, extended mGluR activation (1-5 min) resulted in mammalian target of rapamycin (mTOR)-mediated PP2A suppression and FMRP rephosphorylation. These activity-dependent changes in FMRP phosphorylation were also observed in dendrites and showed a temporal correlation with the translational profile of select FMRP target transcripts. Collectively, these data reveal an immediate-early signaling pathway linking group I mGluR activity to rapid FMRP phosphorylation dynamics mediated by mTOR and PP2A.
Figures




References
-
- Adams DG, Coffee RL, Jr, Zhang H, Pelech S, Strack S, Wadzinski BE. Positive regulation of Raf1-MEK1/2-ERK1/2 signaling by protein serine/threonine phosphatase 2A holoenzymes. J Biol Chem. 2005;280:42644–42654. - PubMed
-
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. - PubMed
-
- Brown V, Small K, Lakkis L, Feng Y, Gunter C, Wilkinson KD, Warren ST. Purified recombinant Fmrp exhibits selective RNA binding as an intrinsic property of the fragile X mental retardation protein. J Biol Chem. 1998;273:15521–15527. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
