Abstract
Efficient americium (Am)/lanthanide (Ln) separation is highly pursued in advanced nuclear fuel cycle for minimizing the long-term radiotoxicity of nuclear waste and maximizing the utilization of nuclear resources. However, such a task is extremely challenging given the chemical similarity between the inherent thermodynamically stable Am(III) and Ln(III) ions. In recent years, interest in Am/Ln separation through oxidizing Am(III) to higher-valent states is reigniting due to theoretically considerable separation efficiency of this approach. This review summarizes the developments in preparation and stabilization of high-valent Am, especially the progresses in the exploitation of the coordination chemistry of these high-valent Am for effective Am/Ln separation. This review aims to inspire the search for efficient redox and coordination systems with more robust Am/Ln separation performance.
Similar content being viewed by others
Introduction
To enable sustainable development of nuclear energy, the concept of an advanced nuclear fuel cycle based on the so-called “partitioning and transmutation†(P/T) strategy has been proposed, by which the minor actinide, such as americium (Am), is partitioned and then converted into short-lived or stable nuclides through transmutation in nuclear reactors or accelerators1,2,3. However, the co-existed lanthanides (Ln) have been proven as problematic for the P/T strategy because of their high neutron-capture cross-sections and great interference in the subsequent actinide transmutation. Accordingly, separating Am from Ln is a critical step in the P/T strategy. Nonetheless, this task remains a long-standing challenge due to the close similarities between Am(III) and Ln(III), particularly in their ionic radii and +3 oxidation state4,5,6,7,8. As a consequence, great efforts have been devoted in the past decades, and two main approaches have been recommended to address this challenge. One extensively exploited approach is using ligands with relatively “softer†N-donor or S-donor atoms, which afford greater covalent bonding interactions to Am(III) than Ln(III) and thus could selectively separate Am(III) from Ln(III) through techniques such as solvent extraction. This method remains the mainstream separation approach widely employed in the nuclear industry due to its high efficiency, continuous operability, scalability, and adaptability to various systems. However, this approach is hampered by some inevitable defects such as poor separation kinetics and instability of ligands, thus restricting its industrial-scale applications6,7,9. Another approach is to make use of the difference in the redox chemistry of Am and Ln. The distinct physicochemical properties of Am in different oxidation states—coordination geometry, redox stability, and reactivity—critically influence its separation behavior (Table 1). While the trivalent Am can be oxidized to the americyl form of high-valent +V or +VI state, lanthanides largely remain in +III state, with a few exceptions in +IV state. By taking advantage of the differences in terms of both charge density and steric configuration between high-valent Am and Ln(III), better Am/Ln discrimination and more efficient mutual separation could be afforded in principle10,11,12,13.
In combination with promising redox-based protocols, multiple techniques including solvent extraction14,15,16,17,18, coprecipitation19,20, and chromatographic method21,22,23,24 have been widely explored for the separation of high-valent Am, especially the +V and +VI americyl ions, from Ln. In spite of this, two prominent issues still impede the practical applications in specific separation scenarios. On one aspect, the redox potentials of high-valent Am/Am(III) couples are quite high (e.g., EAm(VI)-Am(III) = 1.68 V; EAm(V)-Am(III) = 1.73 V; EAm(IV)-Am(III) = 2.62 V vs SCE in 1 M HClO410,11), which results in difficulty in Am(III) oxidation and leaves few options of suitable oxidants9,11,25,26,27,28,29,30,31,32,33. On the other hand, the high-valent Am is very unstable, especially in acidic environments, and can be readily reduced by many species, such as organic solvents, radiolytic products, and free radicals11,14,28,34,35,36,37. The undesired reduction of high-valent Am back to Am(III) leads to a dramatic loss of Am/Ln separation ability, which needs to be effectively overcome in real separation occasions.
In the present review, advances in Am/Ln separation with the aid of high-valent Am have been revisited, with an emphasis on the coordination chemistry of high-valent Am that helped stabilize and separate high-valent Am from Ln. The review is organized to progressively connect fundamental mechanisms to applications: 1. Reagents and methods for Am(III) oxidation (foundational techniques); 2. Coordination-assisted oxidation of Am(III) (ligand-driven stabilization); 3. Separation of Am by oxidation and coordination (redox/coordination synergistic separation). We clarify how breakthroughs in oxidation and coordination chemistry could enable practical separations, offering a framework to evaluate challenges and innovations in Am/Ln separation. We hope this review could inspire more future efforts to search for highly efficient Am/Ln separation systems through Am oxidation.
Reagents and methods for Am(III) oxidation
In light of the large potentials of the Am(VI)/Am(III), Am(V)/Am(III), and Am(IV)/Am(III) redox couples, strong oxidants are required to aid efficient oxidation state control of Am (Fig. 1).
Apart from this, high-valent Am can also be successfully prepared through novel methods such as electrochemical and photochemical oxidation. We herein first summarize the various reagents and methods for the oxidation of Am(III), aiming to provide an overview of the current status in the preparation of high-valent Am, especially Am(V) and Am(VI). Table 2 presents a general summary of the oxidation reagents and methods.
Peroxydisulfate
The first report for the oxidation of Am(III) can be dated back to the 1950s. Asprey et al. unveiled that Am(III) could be oxidized by ammonium peroxydisulfate ((NH4)2S2O8) in 0.2 M HNO3 or HCl solution38. In addition, the final state of Am is closely associated with detailed experimental conditions. Am(III) would be converted to Am(VI) under ambient environment or to Am(V) by heating the acidic solutions39,40,41. These protocols have been repeatedly validated in subsequent studies22,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56. Given the effectiveness of peroxydisulfate in the preparation of Am(V) or Am(VI), studies on the kinetics of the involved reactions were performed. The results indicate that both Am(III) and Am(V) do not react directly with S2O82− but react rapidly with intermediates from the thermal decomposition of S2O82− 57,58,59. Such observations inspired researchers to introduce redox-active component, Ag(I), as a catalyst that helps transfer electrons between S2O82− and Am ions, thereby promoting the oxidation of Am(III). For example, the maximal yield of Am(VI) by the oxidation of S2O82− increased obviously with the presence of AgNO3. On this occasion, Am(III) is considered to be oxidized by SO4− and OH radicals with the aid of catalysis by Ag ion60:
This more efficient approach was extensively applied in numerous following studies27,61,62,63,64,65,66. Despite the effectiveness of S2O82− in Am(III) oxidation, S2O82− may decompose in highly acidic aqueous solutions and generate corrosive sulfate ions5,51,67, which could limit the practical application of peroxydisulfate.
Ozone
Researchers discovered that ozone (O3) could be applied for Am oxidation in 1950s40,41,44,45,47,68,69,70. Despite the standard redox potential of the O2/O3 couple (2.07 V) being much higher than that of the Am(III)/Am(VI) couple (1.69 V), it is difficult to directly oxidize Am(III) to Am(VI), even aided by external heating in an acidic medium49. On this account, it is generally used as an oxidation additive to facilitate the preparation of high-valent Am. Tsushima et al. reported an oxidation rate of approximately 5%/h under photolysis in the presence of ozone at 65 °C in 0.1 M HNO3, where ozone’s main role is to re-oxidize Am(V) to Am(VI) and suppress the reduction effect by decomposing nitrous acid. Although their setup used a 20 mL/min oxygen flow rate with an O3/O2 ratio of ~2.0 × 10−3, no systematic investigation was made regarding the effect of ozone flow rate on oxidation kinetics71. Apart from acidic media, Coleman et al. first oxidized Am(III) by potassium peroxydisulfate (K2S2O8) to generate Am(V) in K2CO3 solution, and then accomplished Am(V)-Am(VI) conversion by O341. Meanwhile, Stephanou et al. reported that O3 can oxidize Am(III) and Am(V) to Am(VI) in carbonate solutions at 25 °C or even lower temperatures40. However, when the temperature increases to 90 °C, the oxidation state of Am will not exceed +V. Thereafter, Gogolev et al. monitored the kinetics of Am(VI) and Am(IV) formation in the case of oxidizing Am(III) by O3 in bicarbonate solution72. It was demonstrated that the yield of Am(VI) decreased with the addition of initial Am content. The substitution of highly radioactive 241Am by 243Am had no obvious impact on the yield and oxidation kinetics of high-valent Am. It can thus be inferred that the radiolysis products generated by 241Am irradiation have little effect on this process. Ozone, as a gaseous oxidant, will not leave significant secondary waste, exhibiting advantages of cleanliness in Am oxidation. However, it is difficult to be further popularized owing to the inherent insufficiency in oxidation ability51,52,72,73.
Perxenate
Holcomb et al. first unraveled that sodium perxenate (Na4XeO6) could convert Am(III) to Am(VI)74,75. Yet for a long period after that, no more reports for the oxidation of Am based on such noble gas compounds until scholars from Russia once again reported it in 2019 and 2020. They observed accumulation of Am(IV) in the Am(III)-containing KHCO3 solution or KHCO3 + K2CO3 solution (pH 8.0–10.5) after exposure to ultraviolet and visible light with wavelength <420 nm. This process began with the photoactivation and was followed by reactions of radical products with Am(III):
The authors believed that xenon trioxide is suitable for the oxidation of Am(III) to Am(IV), assisted by additional photo irradiation in bicarbonate-carbonate media76. Moreover, this work also revealed the oxidation behavior to be strongly dependent on the XeO3 concentration and the pH of the solution. The initial rate of Am(IV) formation showed a near-first-order dependence on XeO3 concentration, while higher pH led to slower reaction rates, presumably due to competition from hydroxide ions and suppression of photoactivation pathways. Furthermore, the resulting Am(IV) species were stabilized via coordination with carbonate ligands, forming spectroscopically distinct complexes. A subsequent study revealed that, upon introducing Na4XeO6·8H2O into a 0.1 M HNO3 solution containing Am(III) and another oxidant of sodium bismuthate (NaBiO3), the solution turned to alkalinous (Ñ€Ð~10) and Am(III) was oxidized to Am(IV) to afford a stable Am(IV)·XeO6 complex77.
Bismuthate
Bismuthate compounds have been widely used for Am(III) oxidation20,23,24,78,79,80,81,82,83,84. It is noteworthy that the oxidation of Am(III) by NaBiO3 will yield different products under different experimental conditions. This section systematically deconstructs the key determinants of Am oxidation selectivity and stabilization, with particular emphasis on the following four interconnected dimensions.
Acid and pH effects
In the 1970s, Hara et al. employed NaBiO3 to oxidize Am(III) and uncovered that the oxidation rate increased with the decrease of acidity25,78,85. It was found that the solution with lower acidity was more conducive to the oxidation of Am(III). Moreover, there was an approximately inverse relationship between the oxidation rate of Am(III) and the acidity of the solution. Besides, Rice et al. achieved selective oxidation of Am(III) to Am(VI) or Am(V) by changing the aqueous media in the presence of NaBiO3. Am(VI) and Am(V) were generated in 1 M H3PO4 and 0.1 M HCl with half-lives of approximately 22 d and 31 d, respectively, while a mixture of Am(III/V/VI) was formed in 1 M acetic acid86. This oxidation state selectivity (Am(V) in HCl versus Am(VI) in H3PO4 versus mixed Am(III/V/VI) in HO2CMe) likely reflects the distinct coordination chemistry of the anions: phosphate strongly stabilizes AmO22+, acetate shows intermediate stabilization leading to mixed oxidation states, while chloride favors AmO2+ formation.
Temperature and kinetics
Temperature is found to be inversely correlated with Am(VI) stability. Am(VI) is only stable at temperatures not exceeding 0 °C25,78,85, while higher temperatures (20–30 °C) accelerated Am(III) oxidation in 0.5–2.0 M HNO3, the resulting Am(VI) exhibited decreasing stability above 10 °C (complete reduction within hours at 30 °C versus weeks at 0 °C), though such an observation is seemingly incorrect according to following studies. Mincher et al. found that quantitative Am(V) was afforded at 80 °C by contacting Am(III)/HNO3 solution with bismuthate powder, while Am(VI) was generated at room temperature11,28. This apparent contradiction may arise from different experimental focuses: Hara et al. studied solution-phase stability of pre-formed Am(VI), while Mincher et al. examined oxidation kinetics under heterogeneous conditions.
Ag-catalyzed oxidation
Inspired by Ag-catalyzed oxidation of Am(III) by peroxydisulfate, Verma et al. incorporated Ag(I) with bismuthate to obtain a new oxidant, AgBiO3. Efficient oxidation of Am(III) exclusively to its pentavalent state was achieved at pH 4 HNO3 solution, and Am(V) was found to be stable for ∼24 h under optimized conditions (pH 4 HNO3, 25 °C, 2.5 mg/mL AgBiO3 in a non-complexing medium)87.
Organic-phase compatibility
Although NaBiO3 has been proven applicable for Am oxidation in aqueous solution, the contact of organic solvent during the biphasic extraction reduces high-valent Am(V/VI) easily14,28,29,34,35. Wang et al. resolved this by designing Bi(V)-incorporated organic solvents that simultaneously oxidize Am(III) and stabilize Am(V) through coordination16,17, as detailed in the “Diglycolamide coordination†and “In situ oxidation and solvent extraction separation of Am†sections.
Copper(III) periodate
Copper(III) periodate has been a burgeoning reagent in recent years. Previous studies have reported that periodate can bind several high-valent transition metals, such as Ni(IV), Cu(III). and Ag(III) to form stable complexes. Among them, the electrical pair of Cu(III/II) in Cu(III) periodate exhibits a high oxidation potential (2.4 V) and can oxidize Np(VI) and Pu(VI) to the heptavalent state in KOH solution88. On this basis, Sinkov et al. investigated the redox chemistry between Cu(III) periodate and Am(III) in HNO3 solution. Over 98% of Am(III) in 3.5 M HNO3 was oxidized to Am(VI) with a Cu(III)/Am(III) mole ratio of 20/1, while the conversion ratio by the oxidation of NaBiO3 is merely 19% under the same conditions. As a consequence, Cu(III) periodate obviously exhibits higher reactivity in the oxidation of Am(III) than bismuthate30. Subsequently, McCann et al. revealed that Cu(III) periodate undergoes self-reduction in HNO3 solution, and this self-reduction effect tends to be more pronounced in highly acidic environments89,90. Such an observation explains the high Cu(III)/Am(III) molar ratio (20:1) required for quantitative Am(VI) generation30.
Electrochemical oxidation
Electrochemical method was used for Am(III) oxidation as early as the 1950s, but it was subsequently ignored for a long time until the 1970s–1980s42,91,92,93. 243Am(IV) had been prepared through electrochemical oxidation of 243Am(III) in multiple carbonate solutions including (NH4)2CO3, Na2CO3, K2CO3 and Cs2CO3. Moreover, applying 1.1 V and 1.3 V to a stable Am(IV)-carbonate solution led to quantitative generation of Am(V) and Am(VI), respectively, as carbonate ions act as strong complex ligands and form soluble complexes with Am(IV) that inhibit hydrolysis and disproportionation94. Myasoedov et al. revealed that the outcomes of electrochemical oxidation of 243Am(III) in 3 M KHCO3-K2CO3 mixtures depended on both the pH of the solution and the anode potential. At a potential of +1.25 V, stable Am(IV), Am(V), and Am(VI) were obtained at pH ranges of 8.2–9.7, >13.5, and 11–12.5, respectively. Meanwhile, formal potentials of Am(IV)/Am(III) and Am(VI)/Am(V) couples in this system were determined as 0.870 ± 0.002 V at pH 8.2 and 0.910 ± 0.003 V at pH 11.3, respectively95. The KHCO3-K2CO3 buffer maintains pH stability through HCO3−/CO32− equilibrium, neutralizing H+ generated during electrolysis95. Researchers from CEA developed the SESAME process based on these findings, combining electrochemical oxidation with lacunary heteropolyanions (LHPA, P2W17O6110− or SiW11O398−) to stabilize Am(IV), followed by oxidation to Am(VI) at controlled potentials. The process uses AgNO3 as a redox mediator, with a critical molar ratio of LHPA/Am <2 to avoid over-complexation and ensure Am(VI) formation91,92. Subsequently, Am(VI) is selectively extracted by 30% TBP in dodecane from 5 M HNO3, achieving >90% recovery in centrifugal contactors, while Cm(III) remains unextracted91,92. In recent years, Dares and colleagues discovered that tin-doped indium oxide electrodes surface-derivatized with terpyridine or dipyrazinylpyridine ligands were able to oxidize Am(III) to Am(V)/Am(VI), providing an alternative approach to access the higher-valent states of Am (Fig. 2)9,31. This approach will be further discussed in the “ Modified electrode†section.
a Am speciation derived from UV-visible spectroscopy during controlled potential electrolysis with a nanoITO|dpp electrode. b Changes in Am species monitored through UV-vis spectra over 11.5 h during bulk electrolysis using a nanoITO|dpp electrode. The figure is recreated from ref. 9 with permission of the Royal Society of Chemistry.
Radiolysis oxidation
The study on the oxidation of Am(III) by means of radiation dates back to the 1980s. The autoradiolytic oxidation of Am(III) to Am(V) in 5 M NaCl solution was explored by Magirius et al. through the combination of pulsed laser-induced photoacoustic spectroscopy and spectrophotometry methods96. They uncovered that Am(III) can be quantitatively converted to Am(V) within a week in the presence of a moderate dose rate (1 Ci/L) of self α-radiation. Their subsequent research revealed that such oxidation can be attributed to α-radiolysis-generated chlorine radicals (Cl·) via Cl− decomposition, driving Am(III) oxidation to stable Am(V) over days97.
A very recent work by Kynman et al. established a unique system for temperature-dependent electron pulse radiolysis to facilitate the radiation-induced formation of Am(IV) in concentrated (6.0 M) HNO3 solution98. In this system, NO3·radicals were generated through HNO3 radiolysis. While NO3· could rapidly oxidize Am(III) to Am(IV) (k = 1.35 × 108 M−1s−1), the radiolysis by-product HNO2 and H2O2 reduce Am(IV) back to Am(III) (lifetime ~16 μs of Am(IV)). Am(III) oxidation is a mechanistically feasible reaction under high HNO3 concentrations and varied temperatures. Nevertheless, the resulting Am(IV) is not sufficiently stable (life of ~16 μs) for further separation test (Fig. 3). Meanwhile, key parameters include organic phases (scavenging radicals), aerobic conditions (enhancing hydroperoxide radicals’ generation), and acid concentration (>4 M HNO3 stabilizes Am(IV) nitrate complexes) are also crucial for the oxidation of Am(III).
Dose-normalized transient absorption spectra from the electron pulse irradiation of Am(III) in aerated 6 M HNO3 at 21 ± 1 °C for several time slices after the electron pulse. The figure is used from ref. 98 with permission of the Royal Society of Chemistry.
Ultrasound oxidation
Up to now, only one study has reported the influence of ultrasound on the redox reactions of Am ions in aqueous solutions99. It was shown that ultrasound accelerated the oxidation of Am(V) to Am(VI) by O3 in 1 M HNO3 at room temperature. In addition, the stability of Am ions in multiple oxidation states under the effect of ultrasound vibrations was investigated by means of the spectrophotometric method. In 0.5 M HClO4 and HNO3 solutions, Am(VI) was rapidly transformed to Am(V), which in turn slowly reduced to Am(III). Furthermore, it was explicit that Am(V) exhibited better stability with the increase of acid concentration. Unfortunately, there are no follow-up reports on this method.
Photochemical oxidation
Numerous studies have shown that photochemical methods (laser or ultraviolet light irradiation) can achieve oxidation state control of metal ions76,100. Photochemical oxidation of Am(III) in dilute HNO3 solution with the aid of O3 was studied by Tsushima et al. They disclosed that Am(III) in 0.1 M HNO3 can be photo-oxidized to its hexavalent state by a deuterium lamp with emitting lines below 170 nm71. Besides, Sheridan et al. used a TiO2 electrode to assist the photocatalytic conversion of Am(III) to Am(VI)32. The results showed that the oxidation occurs through photoelectrochemically generated adsorbed hydroxyl radicals and/or direct electron transfer between the excited-state electrode and Am(III). An electrochemically irreversible but chemically reversible electrochemical process at 1.60 V vs SCE was assigned to the one-electron transfer of Am(VI/V) couple (Fig. 4). Recently, Matsuda et al. showed resonance-enhanced multiphoton charge transfer in actinide complexes, which results in specific oxidation state control of the element owing to the distinct electronic spectra arising from resonant transitions in f-orbitals33. Additionally, they found that the coordination of NO3− is necessary for promoting the oxidation process, which is the first finding relevant to the primary process of photoexcitation via resonant transitions of Am.
Proposed photoelectrolysis scheme for the generation of Am(VI) from Am(III). The figure is used from ref. 32 with permission of the American Chemical Society.
Coordination-mediated oxidation and stabilization of high-valent Am
The oxidation of Am(III) to higher-valent states is challenging due to the high redox potentials of Am(IV/V/VI)/Am(III) couples. This section explores how coordination can mitigate these barriers by stabilizing oxidized Am species. Ligands such as carbonates, polyoxometalates, and terpyridine derivatives can modify the inner coordination sphere of Am, lowering effective redox potentials and inhibiting reduction pathways. We first discuss how these ligands thermodynamically favor oxidation, followed by describing their role in separation processes. As a matter of fact, this approach has been well demonstrated for the oxidation state control of other actinides such as neptunium (Np) and plutonium (Pu)101,102,103.
Carbonate-bicarbonate coordination
Carbonate is a well-known anionic ligand capable of forming strong complexes with actinide ions under neutral or alkaline conditions; thus, the carbonate medium provides a unique environment to modify the redox chemistry of Am. In 1983, Bourges et al. discovered the coexistence of Am in four oxidation states in a mixed Na2CO3-NaHCO3 medium104. Such an occurrence is completely different from that in acidic environments, suggesting that the coordinating anion in different media basically affects the redox properties of Am. Specifically, the values of the apparent normal potentials of Am(VI)/Am(V) and Am(IV)/Am(III) couples in Na2CO3-NaHCO3 media with total [CO32− + HCO3−] concentrations ranging from 1.2 M to 2.3 M (pH ranges from 9.5 to 10.5) were explicitly measured with the combination of potentiometric titration and spectrophotometric method. The Am(VI)/Am(V) couple (E ~ 0.975 ± 0.01 V vs. NHE) is independent of CO32− concentration, while the potential of the Am(IV)/Am(III) couple decreases monotonically as CO32− concentration increases (E ~ 0.924 ± 0.01 V vs. NHE to [CO32−] = 1 M). The exchange of two CO32− during the transition from the carbonate form of Am(III) to that of Am(IV) was thus identified according to the variation (127 mV) in the normal potential of the Am(IV)/Am(III) couple as a function of log[CO32−]. Meanwhile, the difficulty in obtaining pure Am(IV) product in CO32− media with pH over 11 can be ascribed to the disproportionation of Am(IV), rather than the similarity of the apparent normal potentials between Am(IV)/Am(III) and Am(VI)/Am(V) couples.
Triphenylarsine oxide coordination
A spectroscopic and electrochemical study of f-block elements by Payne et al. uncovered that the redox behaviors of representative lanthanides and Am underwent significant changes when bound with triphenylarsine oxide (TPAsO)105. A cyclic voltammogram was obtained for Am in nitrate-TPAsO-acetonitrile mixed media with LiNO3 as supporting electrolyte, and a shift of about 1.7 V in potential favoring Am(IV) generation was observed for the Am(IV)/Am(III) couple. Accordingly, the stabilization of Am(IV)-TPAsO species in acetonitrile is greater than that of Am(IV)-carbonate species in aqueous solution. It should be noted that the solvent itself also plays an important role in the oxidation state control. Several Ln(III)-phosphine and Ln(III)-arsine oxide complexes in ethanol have been studied previously, but only the trivalent species were observed in each case106,107,108. Hence, both the TPAsO ligand and the acetonitrile solvent, rather than just the ligand itself, are important in these redox chemistry studies. The steric shielding effect from the bulky phenyl groups should be more remarkable for the M(IV) complex than the corresponding M(III) complex due to the smaller ionic radius of M(IV), indicating the M(IV) complex is extremely stable once it is generated (M represents Ln or Am ions). This could be an important factor in stabilizing and characterizing other atypical oxidation states of actinides, both in solid and liquid states.
Polyoxometalate coordination
Polyoxometalate compounds (POMs) are a well-known class of nanoscale, inorganic, metal-oxo clusters assembled from simple MOx units (M = V, Mo, W; x = 4-6)109,110,111,112. POMs have shown a notable impact on metal ions’ redox potentials. Litvina et al. took the lead to conduct a systematic exploration concerning Am oxidation by (NH4)2S2O8/Ag mixture in mineral acids in the presence of unsaturated potassium phosphotungstate (K10P2W17O61)113. The lower redox potential of the Am(IV)/Am(III) couple (E0 = 1.52 V) with the coordination of K10P2W17O61 as compared to that of the “free†state couple (E0 = 2.62 V)10 allows relatively easy preparation of Am(IV) in HNO3, H2SO4, and HClO4 solutions. The mechanism of Am(III) oxidation is that S2O82− initially oxidizes Ag(I) to a high-valent state and then Ag(II) in turn oxidizes Am(III) to Am(IV). All the newly formed Am(IV) is coordinated by excessive K10P2W17O61 and stabilized for a long time. Am(III) would also be oxidized to Am(IV) in the presence of stoichiometrically deficient K10P2W17O61, but the non-coordinated Am(IV) spontaneously disproportionates to Am(III) and Am(VI). Am(VI) is produced only as a result of Am(IV) disproportionation. The stability constants of Am(III) and Am(IV) complexes with representative polyoxometalates in 1 M HNO3 were determined by Picart et al.114. The logβ11 and logβ12 values of Am(III)-P2W17O6110− complexes are 2.7 and 4.8, respectively, while the corresponding values of Am(IV)-P2W17O6110− complexes are much higher (19.3 and 22.9, respectively). Likewise, the stability constants of Am(III/IV) complexes with another silicotungstate compound (SiW11O398−) are also determined to be 4.4 (logβ11) and 6.7 (logβ12) for Am(III)-SiW11O398− and 21.3 (logβ11) and 26.1 (logβ12) for Am(IV)-SiW11O398−. Such prominent discrepancies in stability constants basically contribute to the shifting in redox potential of the Am(IV)/Am(III) couple. Supported by this theory, researchers from Russia and France accomplished efficient oxidation state control and separation of Am with a combination of electrochemical method115,116,117,118.
Modified electrode
Dares and coworkers reported in 2015 a scheme of using the terpyridine-modified high-surface area, tin-doped indium oxide electrode to coordinate Am, thereby facilitating the oxidation of Am(III) to Am(V) and Am(VI) (Fig. 5)31. A potential of 1.8 V versus the saturated calomel electrode was applied, and it led to 0.7 V lower shifting of the one-electron transfer oxidation of Am(III) to Am(IV) in 1 M nitric acid with an intrinsic redox potential of 2.6 V. The mechanism appears to involve both surface binding of Am(III) and oxidation to Am(IV), followed by further oxidation to Am(V). Dares et al. later further altered the surface ligand to dipyrazinylpyridine and explored its effect on the oxidation of Am(III)9. Their findings indicated that only Am(V) is observed at a potential of 1.8 V. Am(III) was first oxidized to Am(V) and subsequently converted to Am(VI) by increasing the potential to 2.0 V. When the applied potentials were over 2.0 V, Am(III) was oxidized to Am(V), accompanied by the reduction of Am(VI) to Am(V). The latter reduction might be ascribed to the increased rate of hydrogen peroxide generation from water molecules at the electrode at these high potentials. These studies offer an opportunity to acquire high-valent state Am ions in non-complexing media for the study of the associated coordination chemistry and probing more efficient separation systems.
a Molecular structure of p-tpy on the surface of an ITO particle with the protonation state depicted as expected in neutral pH. b Am speciation as measured by visible spectroscopy during controlled potential electrolysis using a p-tpy-derivatized ITO electrode. c Visible spectra of species before and after 13 h of electrolysis with highlighted speciation changes. The figure is recreated from ref. 31 with permission of The American Association for the Advancement of Science.
Diglycolamide coordination
Owing to the strong extraction ability of a diglycolamide ligand (N,N,N’,N’-tetraoctyl diglycolamide, TODGA) toward trivalent and tetravalent f-block elements, TODGA has great potential to modify the redox behavior of Am(III). Unlike previous studies imposing oxidation in aqueous solution, Wang et al. recently established a unique system with Bi(V) incorporated in an organic phase, accomplishing efficient generation and stabilization of Am(V) either in the organic phase (without the presence of aqueous phase) or in the aqueous phase (with both organic and aqueous phases present)16,17,18. Bi(V)-incorporated solvent was prepared by solvent extraction of Bi(V) from acidic solutions by TODGA in dodecane. Am(III) was also separately extracted into dodecane by TODGA. When the two organic solutions were mixed together, Am(V) was generated immediately and could be stabilized for tens of minutes. Large-scale quantum mechanics/molecular mechanics (QM/MM) molecular dynamics (MD) simulations and density functional theory (DFT) calculations were conducted to elucidate the oxidation mechanism. The results revealed that the unique redox and coordination environment of the Bi(V)/TODGA organic solvent plays a vital role in the formation of Am(V). Furthermore, when there was an aqueous phase (nitric acid solution) present, Am(V) will transfer into the aqueous phase due to the weak coordination between Am(V) and TODGA. And the system will form a compensative loop to stabilize Am(V) in the aqueous phase, which is a key feature that helps separate Am from lanthanides, as will be discussed later in the following section (Fig. 6).
a Scheme for efficient generation and stabilization of Am(V) with the initial oxidation state of Am in the aqueous phase is +â…¢ in a biphasic cycle by incorporating Bi(V) species into the organic phase. b Scheme for efficient generation and stabilization of Am(V) with the initial oxidation state of Am in the aqueous phase is +â…¥ in a biphasic cycle by incorporating Bi(V) species into the organic phase. The figures are used from ref. 17 and ref. 18 with permission of the American Chemical Society and the Royal Society of Chemistry.
Am/Ln separation by oxidation and coordination
As aforementioned, high-valent Am ions, especially the +V and +VI ions in americyl form, are significantly different from the trivalent lanthanides in terms of both steric configuration and ionic charge, thus providing a strong chemical basis for their mutual separation through selective coordination. Till now, there are quite a few methods that could realize quantitative oxidation of Am(III) to high-valent Am. However, on the separation side, the prepared high-valent Am ions are usually subject to fast reduction, causing sharp deterioration in Am separation efficiency and impeding the real application of these oxidation-based separation methods. How to stabilize the high-valent Am ions during the separation process remains a great challenge despite some encouraging progress having been made recently. In this section, we will first revisit the traditional separation efforts in which Am(III) was oxidized in aqueous solution and then separated with techniques such as solvent extraction and precipitation. Then, newly developed separation schemes with an emphasis on stabilizing the high-valent Am ions during the separation process were reviewed and discussed.
Traditional solvent extraction separation of pre-oxidized Am
Since the 1990s, Kamoshida et al. have conducted a series of investigations on the Am/Ln separation through a stepwise approach. The methodology involves the initial oxidation of Am(III) to Am(VI) using peroxodisulfate, followed by the selective separation of Am(VI) from Ln(III) using certain ligands via solvent extraction26. The experimental results demonstrated that the extraction of Am(VI) using undiluted tributylphosphate (TBP) yielded a distribution coefficient of Am(VI) exceeding 1 and afforded an Am/Nd separation factor (SFAm/Nd) of 87 ± 9. Subsequently, they attempted to optimize the separation efficiency by introducing specific coordinating agents into the aqueous phase, which stabilized Am(VI) through selective complexation27. With the stabilization effect of NH4H2PO4, the Am/Nd separation factor was improved to 120. However, a significant challenge remains in the inherent instability of the Am(VI) ion, particularly when exposed to reductive organic solutions during solvent extraction processes. This instability leads to the rapid reduction of Am(VI) to Am(V) or Am(III), thereby compromising the selectivity of the separation. For instance, studies by Mincher et al. revealed that freshly prepared Am(VI) undergoes significant reduction within tens of seconds or minutes upon contact with the organic phase, severely limiting subsequent separation operations14,28,29,34. Additionally, the coexisting Ce(III) can be oxidized to the extractable Ce(IV) state, which interferes with the effective separation of Am from lanthanides35. To circumvent the issue of Ce co-extraction, researchers explored an alternative approach of oxidizing Am(III) to the non-extractable Am(V) state using (NH4)2S2O8. In this case, favorable separation efficiencies with SFEu/Am and SFCe/Am values of approximately 50 were achieved with the synergistic extraction of octylphenyl-N,N-diisobutylcarbamoylmethylphosphine oxide (CMPO) and TBP55. In recent years, researchers have investigated the group separation of actinides (An) and lanthanides (Ln) by oxidizing critical actinide elements, including U, Np, Pu, and Am, to the +VI state. This process involves the extraction of An(VI) into the organic phase while retaining Ln(III/IV) in the aqueous phase81,90. With this regard, considerable single-stage SFAn/Ln values ranging from 102 to 105 were afforded with the aid of selective extraction by a branched-chain extractant, 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester (EHEHPA)12. However, short contact time in extraction still has to be precisely controlled to achieve appreciable separation. These advancements highlight the ongoing efforts to address both the challenges of high-valent Am stability and extraction selectivity in Am/Ln separation on the basis of oxidation and coordination.
Selective coordination and precipitation of high-valent Am
Given the instability of high-valent Am in the presence of organic compounds, alternative solid-liquid separation methods, i.e., selective coordination and precipitation, have been developed to address this challenging task. Shehee et al. selectively precipitated lanthanides in the form of sodium lanthanide sulfate salts from the higher-valent states of Am(VI) produced by Na2S2O8 oxidation52,53. The oxidized Am species predominantly remained in a non-complexed state in the supernatant phase. Parallel studies involving oxidized U, Np, and Pu solutions supported the conclusion that Am(VI) exhibits sufficient stability to allow the separation to be completed within a reasonable time frame. The retention percentage gave an Am/Eu separation factor of ~11 in a single contact. Besides, the feasibility of recovering co-crystallized Np, Pu, and Am from a uranyl nitrate hexahydrate crystalline phase has been explored20. Progressive dissolution studies were conducted on samples of hexavalent Np, Pu, or Am co-crystallized with UO2(NO3)2·6H2O. The results revealed a linear relationship between the amount of dissolved actinides and the volume of added HNO3, indicating a homogeneous distribution of transuranic species throughout the crystalline solid. A series of five “crystallization-dissolution-recrystallization†cycles of UO2(NO3)2·6H2O from HNO3 solution was successfully performed in the presence of Am(VI). Throughout these cycles, the behavior of Am(VI) closely mirrored that of U(VI), with negligible reduction of Am(VI) to Am(III) observed between each recrystallization step. These findings underscore the potential of a group hexavalent actinide co-crystallization approach for the recycling of spent nuclear fuel.
Chromatographic Am/Ln separation by oxidant materials
Chromatography was also employed for the separation of high-valent Am and Ln. Burns et al. investigated the sorption behaviors of both Am(III) and Am(V) using four M(IV) phosphate-phosphonate ion exchange materials in a pH 2 HNO3 solution54. High selectivity for the trivalent Am was observed with Kd values of 6 × 105 mL/g, while that of Am(V) was much lower. An effective method of synthesizing and stabilizing pentavalent Am, achieving a stability period exceeding 24 h in an acidic medium, was developed with the combination use of Na2S2O8 and Ca(ClO)2. Additionally, a demonstration based on Na-Sn-hybrid ion exchanger afforded separation factors of 30 ± 10 for Am/Nd and 60 ± 20 for Am/Eu, respectively. To streamline the separation operations, several redox-active chromatographic materials incorporating oxidants such as NaBiO3 and AgBiO3 have been prepared, enabling simultaneous oxidation and separation24,87. In the studies by Verma et al., the ion exchange of Ln(III) with interlayer Na(I) in NaBiO3 was validated through an ionic strength variation experiment83. The experimental observations indicated the Am(III) → Am(VI) conversion during the dissolution of NaBiO3·xH2O (x = 2–3) even at pH∼1. Such a mechanism paves the way towards designing more efficient materials for Am/Ln separation. Very recently, on the basis of the aforementioned results, Labb et al. extended the application of NaBiO3 chromatography to analyze the adsorption, kinetics, and elution behaviors of U, Pu, and Eu84. Separation factors exceeding 200 with rapid kinetics were observed at dilute HNO3 solutions, achieving complete An/Ln group separation. Such an investigation highlighted the great potential of a NaBiO3-based separation following the TRUEX process.
In situ oxidation and solvent extraction separation of Am
As discussed in the “Diglycolamide coordination†section, Wang et al. established a novel scheme to oxidize Am(III) to Am(V) in situ in an organic solvent by pre-incorporating the oxidant Bi(V) into the solvent with TODGA as a functional ligand17. The prepared Am(V) can be stabilized for a long duration either in the organic phase or in an aqueous phase in the presence of a Bi(V)-incorporated organic phase. When both Am and lanthanides are present in the biphasic system, the oxidized Am species, Am(V), will disassociate with TODGA and transfer to the aqueous solution owing to the intrinsic weak affinity between pentavalent actinyl ion and TODGA. On the other hand, TODGA exhibits a strong coordination ability to trivalent and tetravalent Ln, thus retaining these Ln ions in the organic phase. By taking advantage of such a significant difference in coordination behavior, Am and Ln can be well separated in the aqueous phase and organic phase, respectively (Fig. 7). Through a single biphasic contact, an Eu/Am separation factor of over 104 can be achieved. Other than the very short contact time required for appreciable Am/Ln separation in traditional methods, as discussed previously in the “Traditional solvent extraction separation of pre-oxidized Am†section, the high separation efficiency could last for a long time, up to hours of contact in this new approach. Moreover, the efficient separation can be retained over a very wide range of nitric acid concentrations (1-14 M HNO3). Dong et al. recently further modified this method by first oxidizing Am(III) to Am(VI) in nitric acid solution and then contacting this Am(VI) solution with Bi(V)-incorporated TODGA/n-dodecane organic solution18. In this case, Am(V) can be even more efficiently generated through the reduction of Am(VI) and oxidation of Am(III). When both Am(III) and Ln(III) are present in the initial aqueous solution, Am and Ln can then be separated with record-high separation factors (>105) through a single biphasic contact using this modified method. It should be noted that the intrinsic Ln(III,IV)/Am(V) selectivity by TODGA coordination is still the core driving force for the separation.
a The dynamic Am(III)/Am(V) recycle for the generation and stabilization of Am(V) in the biphasic system with the Bi(V)-containing organic phase. b The reactions of Ln in the same system (Note: only Ce could be oxidized from III to IV in this system). The species in the thick dashed frame represent the final species in the system after equilibrium. The figure is copied from ref. 17 with permission of the American Chemical Society.
Polyoxometalate coordination and separation of Am
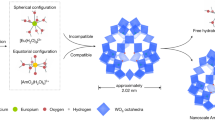
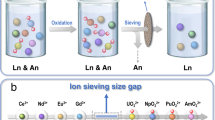
The coordination chemistry of POMs with transuranium elements has been investigated on quite a few occasions, and, as aforementioned, POMs can be used to modify the redox potentials of Am and facilitate Am(III) oxidation. However, direct utilization of POMs for Am separation has been very limited. Myasoedov et al. investigated the extraction of Am(IV) by secondary amines from HNO3 and H2SO4 solutions in the presence of K10P2W17O6192. Am(IV) in the POM-containing mineral acids can be quantitatively extracted, and the compositions of the extracted compounds were also determined. Meanwhile, (NH4)10P2W17O61 was also synthesized and applied to the stabilization and separation of Am. It was revealed that Am(VI) could be extracted by TBP in the presence of POM64. The distribution ratio of Am was 4 when extracted from 1 M HNO3, and its separation factor from Nd(III) was 50. Very recently, Zhang et al. synthesized a lacunary POM {Se6W45} equipped with a vacant equatorial donor site precisely matching the common pentagonal bipyramid coordination geometry of an actinyl ion, and the site is unsuitable for binding Ln(III) ions (Fig. 8)119. The precise and strong coordination of {Se6W45} with Am(VI) could help stabilize the hexavalent Am ions to an unprecedented level, with only 0.67% of the Am(VI) ions being reduced in the presence of POM clusters over a period of 24 h. Moreover, the POM can efficiently discriminate americium and lanthanides with a large size difference (~2 nm vs 0.45 nm) between their coexisting chemical species, which leads to a new separation method based on an industrial ultrafiltration technique. The separation of nanoscale Am(VI)-POM clusters from hydrated Ln ions by a fine-pored membrane is very efficient, with a once-through Am/Eu separation factor over 750. This separation efficiency is obviously higher than the system by sieving of hydrated Am(VI) and Ln(III) ions, which have a very limited size difference, through a graphene oxide membrane13.
a Schematic illustration of the frame of the ultrafiltration separation of nanoscale Am(VI)-POM clusters from Ln. b Demonstration of Am separation through oxidation, complexing, and ultrafiltration using POM as a complexant. c Depiction of actinide group separation and ultrafiltration separation results of U(VI), Np(VI), Pu(VI), Am(VI), and Eu(III). d Comparison with the representative Am(VI)-based separation performances on the recovery rate of Am and separation factor. The figure is recreated from ref. 119 with the permission of Springer.
Summary and outlook
Am plays a critical role in the nuclear fuel cycle. Its efficient separation could facilitate the safe treatment of nuclear waste and help maximize the utilization of nuclear resources. Yet the high radiotoxicity, rare availability, and tricky chemistry of this transuranic element make its separation study lag behind most other metal ions. In particular, the separation of Am from its 4f analogs, the lanthanides, poses a great challenge due to the extremely similar chemical properties between the two groups of elements. With most of the research efforts devoted to the development of functional ligands bearing soft donor atoms such as N and S that could discriminate Am from Ln based on slight differences in f-orbital diffusion, separation attempts by taking advantage of the redox chemistry of Am are less explored but are believed to be important alternatives to further boost the separation efficiency. In this review, we first revisited the reagents and methods used over the past decades for the oxidation of Am(III) to high-valent states, and then summarized the latest advances in Am/Ln separation by means of oxidation state control of Am, with a special focus on exploiting the coordination chemistry of high-valent Am.
Currently, Am(III) has been successfully oxidized to three high-valent states, i.e., IV, V, and VI, and all of which have been exploited for Am/Ln separation. Nevertheless, the separation efficiency and applicable conditions varied a lot among these oxidation states. For Am(IV), its preparation and stabilization usually rely on strong coordination by anionic ligands such as F−120,121,122 and carbonate45,47,68,70,94,122, which are not the favorable media in nuclear waste treatment, with acidic conditions being predominant. The reported separation efficiency of Am(IV) from lanthanides was also not satisfactory, especially when we consider the presence of Ce(IV) under an oxidizing environment. Ce is one of the major lanthanides coexisting with Am in spent nuclear fuel, and it will exist in the +IV oxidation state in the presence of strong oxidants. For Am(V), either in terms of steric configuration or ionic charge, it presents the biggest difference from trivalent and tetravalent lanthanides, therefore potentially enabling the highest Am/Ln separation efficiency through selective coordination. Unfortunately, oxidation and separation studies involving Am(V) have been seldom reported, probably due to the difficulty in quantitative preparation of Am(V), which is usually subject to disproportionation. Until very recently, with the synergistic assistance of oxidation and coordination in an organic solvent, the quantitative preparation and robust stabilization of Am(V) under ambient conditions were realized, and extraordinary Am/Ln separation efficiency was achieved. Most of the studies related to Am oxidation and separation have been devoted to Am(VI), so far, the highest oxidation state of Am. Quantitative oxidation of Am(III) to Am(VI) has been realized using a variety of oxidants, but separation studies with Am(VI) have encountered significant challenges due to the easy reduction of Am(VI) through self-radiolysis or by organic reagents used in the separation process. Recent progress using inorganic polyoxometalates to coordinate, stabilize, and separate Am(VI) offers new opportunities in this area. One more point is that the oxidation state of Am is the preferable one in separation, depending on specific scenarios. If we consider the separation of only individual Am from Ln, Am(V) is obviously a good choice as Am(V) is the most chemically different form from Ln(III) and Ln(IV). But if the aim is group separation of actinides, including U, Np, Pu, and Am from Ln, which has been proposed as an advanced concept in nuclear fuel cycle, Am(VI) would be a better choice since all the concerned actinides can be more easily retained in the hexavalent other than the pentavalent state.
Motivated by the great potential of oxidation-based Am separation and inspired by the enormous efforts of previous studies, we could foresee more attempts to apply Am redox chemistry in Am separation to facilitate nuclear waste treatment and nuclear resources utilization. In these ongoing and incoming attempts, the coordination chemistry of Am would be a key to unlock the gateway to high efficiency in oxidation and separation. Precise coordination would not only help control the redox potential of Am but also establish the chemical scheme for separation. Nevertheless, coordination chemistry of Am in different oxidation states, especially these rare-accessible high-valent states, is complex, and great efforts and multidisciplinary contributions from researchers in different areas such as organic chemistry, analytical chemistry, and computational chemistry are required to address the challenges ahead.
References
Salvatores, M. Nuclear fuel cycle strategies including partitioning and transmutation. Nucl. Eng. Des. 235, 805–816 (2005).
Salvatores, M. & Palmiotti, G. Radioactive waste partitioning and transmutation within advanced fuel cycles: achievements and challenges. Prog. Part. Nucl. Phys. 66, 144–166 (2011).
Gonzalez-Romero, E. M. Impact of partitioning and transmutation on the high level waste management. Nucl. Eng. Des. 241, 3436–3444 (2011).
Lewis, F. W., Hudson, M. J. & Harwood, L. M. Development of highly selective ligands for separations of actinides from lanthanides in the nuclear fuel cycle. Synlett 2011, 2609–2632 (2011).
Hudson, M. J., Harwood, L. M., Laventine, D. M. & Lewis, F. W. Use of soft heterocyclic N-donor ligands to separate actinides and lanthanides. Inorg. Chem. 52, 3414–3428 (2013).
Panak, P. J. & Geist, A. Complexation and extraction of trivalent actinides and lanthanides by triazinylpyridine N-donor ligands. Chem. Rev. 113, 1199–1236 (2013).
Leoncini, A., Huskens, J. & Verboom, W. Ligands for f-element extraction used in the nuclear fuel cycle. Chem. Soc. Rev. 46, 7229–7273 (2017).
Xu, L., Yang, X. F., Zhang, A. Y., Xu, C. & Xiao, C. L. Separation and complexation of f-block elements using hard-soft donors combined phenanthroline extractants. Coord. Chem. Rev. 496, 215404 (2023).
Lopez, M. J., Sheridan, M. V., McLachlan, J. R., Grimes, T. S. & Dares, C. J. Electrochemical oxidation of trivalent americium using a dipyrazinylpyridine modified ITO electrode. Chem. Commun. 55, 4035–4038 (2019).
Morss, L. R., Edelstein, N. M. & Fuger J. The Chemistry of the Actinide and Transactinide Elements (Springer, Netherlands, 2006).
Runde, W. H. & Mincher, B. J. Higher oxidation states of americium: preparation, characterization and use for separations. Chem. Rev. 111, 5723–5741 (2011).
Dong, X. et al. Group separation of hexavalent actinides from lanthanides through selective extraction by sterically hindered 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester. Ind. Eng. Chem. Res. 61, 17175–17182 (2022).
Wang, Z. et al. Ion sieving in graphene oxide membrane enables efficient actinides/lanthanides separation. Nat. Commun. 14, 261 (2023).
Mincher, B. J., Martin, L. R. & Schmitt, N. C. Diamylamylphosphonate solvent extraction of Am(VI) from nuclear fuel raffinate simulant solution. Solvent Extr. Ion-. Exch. 30, 445–456 (2012).
Taylor, R. Reprocessing and Recycling of Spent Nuclear Fuel (Elsevier, Netherlands, 2015).
Wang, Z., Dong, X., Yan, Q., Chen, J. & Xu, C. Separation of americium from curium through oxidation state control with record efficiency. Anal. Chem. 94, 7743–7746 (2022).
Wang, Z. P. et al. Ultra-efficient americium/lanthanide separation through oxidation state control. J. Am. Chem. Soc. 144, 6383–6389 (2022).
Dong, X., Hao, H. X., Chen, J., Wang, Z. P. & Xu, C. Redox stabilization of Am(V) in a biphasic extraction system boosts americium/lanthanides separation efficiency. Chem. Sci. 15, 2118–2122 (2024).
Stephanou, S. E. & Penneman, R. A. Observations on curium valence states; a rapid separation of americium and curium. J. Am. Chem. Soc. 74, 3701–3702 (1952).
Einkauf, J. D. & Burns, J. D. Recovery of oxidized actinides, Np(VI), Pu(VI), and Am(VI), from cocrystallized uranyl nitrate hexahydrate: a single technology approach to used nuclear fuel recycling. Ind. Eng. Chem. Res. 59, 4756–4761 (2020).
Hulet, E. K. An investigation of the extraction-chromatography of Am(VI) and Bk(IV). J. Inorg. Nucl. Chem. 26, 1721–1726 (1964).
Burns, J. D., Shehee, T. C., Clearfield, A. & Hobbs, D. T. Separation of americium from curium by oxidation and ion exchange. Anal. Chem. 84, 6930–6932 (2012).
Richards, J. M. & Sudowe, R. Separation of americium in high oxidation states from curium utilizing sodium bismuthate. Anal. Chem. 88, 4605–4608 (2016).
Wang, N. et al. Cerium separation with NaBiO3 nanoflower material via an oxidation adsorption strategy. J. Mater. Chem. A 8, 7907–7913 (2020).
Hara, M. & Suzuki, S. Oxidation of americium(III) with sodium bismuthate. J. Radioanal. Chem. 36, 95–104 (1977).
Kamoshida, M. & Fukasawa, T. Solvent extraction of americium(VI) by tri-n-butyl phosphate. J. Nucl. Sci. Technol. 33, 403–408 (1996).
Koma, Y., Aoshima, A., Kamoshida, M. & Sasahira, A. Extraction of Am(VI) from nitric acid solution containing phosphate anion by TBP. J. Nucl. Sci. Technol. 39, 317–320 (2002).
Mincher, B. J., Martin, L. R. & Schmitt, N. C. Tributylphosphate extraction behavior of bismuthate-oxidized americium. Inorg. Chem. 47, 6984–6989 (2008).
Martin, L. R., Mincher, B. J. & Schmitt, N. C. Extraction of americium(VI) by a neutral phosphonate ligand. J. Radioanal. Nucl. Chem. 282, 523–526 (2009).
Sinkov, S. I. & Lumetta, G. J. Americium(III) oxidation by copper(III) periodate in nitric acid solution as compared with the action of Bi(V) compounds of sodium, lithium, and potassium. Radiochim. Acta 103, 541–552 (2015).
Dares, C. J., Lapides, A. M., Mincher, B. J. & Meyer, T. J. Electrochemical oxidation of 243Am(III) in nitric acid by a terpyridyl-derivatized electrode. Science 350, 652–655 (2015).
Sheridan, M. V., Gonzalez-Moya, J. R., McLachlan, J. R., Grimes, T. S. & Dares, C. J. Photocatalytic conversion of Am(III) to Am(VI) using a TiO2 electrode. ACS Appl. Energy Mater. 4, 11854–11857 (2021).
Matsuda, S. et al. Marking actinides for separation: resonance-enhanced multiphoton charge transfer in actinide complexes. Sci. Adv. 8, eabn1991 (2022).
Mincher, B. J., Schmitt, N. C. & Case, M. E. A TRUEX-based separation of americium from the lanthanides. Solvent Extr. Ion. Exch. 29, 247–259 (2011).
Mincher, B. J. et al. Characterizing diamylamylphosphonate (DAAP) as an americium ligand for nuclear fuel-cycle applications. Solvent Extr. Ion. Exch. 32, 153–166 (2014).
Grimes, T. S. et al. Kinetics of the autoreduction of hexavalent americium in aqueous nitric acid. Inorg. Chem. 56, 8295–8301 (2017).
Horne, G. P. et al. Effect of ionizing radiation on the redox chemistry of penta- and hexavalent americium. Inorg. Chem. 58, 8551–8559 (2019).
Asprey, L. B., Stephanou, S. E. & Penneman, R. A. A new valence state of americium, Am(VI). J. Am. Chem. Soc. 72, 1425–1426 (1950).
Werner, L. B. & Perlman, I. The pentavalent state of americium. J. Am. Chem. Soc. 73, 495–496 (1951).
Stephanou, S. E., Nigon, J. P. & Penneman, R. A. The solution absorption spectra of americium (III), (V), and (VI). J. Chem. Phys. 21, 42–45 (1953).
Coleman, J. S. et al. Purification of Gram Amounts of Americium (Los Alamos National Lab. (LANL), Los Alamos, 1955).
Asprey, L. B., Stephanou, S. E. & Penneman, R. A. Hexavalent Americium. J. Am. Chem. Soc. 73, 5715–5717 (1951).
Keenan, T. K. Americium and curium. J. Chem. Educ. 36, 27–31 (1959).
Penneman, R. A., Coleman, J. S. & Keenan, T. K. Alkaline oxidation of americium; preparation and reactions of Am(IV) hydroxide. J. Inorg. Nucl. Chem. 17, 138–145 (1961).
Coleman, J. S., Keenan, T. K., Jones, L. H., Carnall, W. T. & Penneman, R. A. Preparation and properties of americium (VI) in aqueous carbonate solutions. Inorg. Chem. 2, 58–61 (1963).
Moore, F. L. Extraction chromatographic method for rapid separation of americium from other transuranium elements. Anal. Chem. 40, 2130–2133 (1968).
Burney, G. A. Separation of americium from curium by precipitation of K3AmO2(CO3)2. Nucl. Appl. 4, 217–221 (1968).
Fardy, J. J. & Buchanan, J. M. Separation of oxidised americium from trivalent transplutonium elements and lanthanides by solvent extraction. J. Inorg. Nucl. Chem. 38, 149–154 (1976).
Schulz, W. W. The Chemistry of Americium (Atlantic Richfield Hanford Co., Richland, Washington State, 1976).
Reed, W. A., Garnov, A. Y., Rao, L. F., Nash, K. L. & Bond, A. H. Oxidative alkaline leaching of americium from simulated high-level nuclear waste sludges. Sep. Sci. Technol. 40, 1029–1046 (2005).
Martin, L. R., Mincher, B. J. & Schmitt, N. C. Understanding the Chemistry of Uncommon Americium Oxidation States for Application to Actinide/Lanthanide Separations (Idaho National Lab. (INL), Idaho Falls, 2007).
Shehee, T. C., Martin, L. R. & Nash, K. L. Solid-liquid separation of oxidized americium from fission product lanthanides. Mater. Sci. Eng. 9, 012066 (2010).
Shehee, T., Martin, L. R., Zalupski, P. R. & Nash, K. L. Redox-based separation of americium from lanthanides in sulfate media. Sep. Sci. Technol. 45, 1743–1752 (2010).
Burns, J. D., Borkowski, M., Clearfield, A. & Reed, D. T. Separation of oxidized americium from lanthanides by use of pillared metal (IV) phosphate-phosphonate hybrid materials. Radiochim. Acta 100, 901–906 (2012).
Mincher, B. J., Schmitt, N. C., Schuetz, B. K., Shehee, T. C. & Hobbs, D. T. Recent advances in f-element separations based on a new method for the production of pentavalent americium in acidic solution. RSC Adv. 5, 27205–27210 (2015).
Kulyako, Y. M. et al. Separation of Am and Cm by extraction from weakly acidic nitrate solutions with tributyl phosphate in isoparaffin diluent. Radiochemistry 60, 18–22 (2018).
Ermakov, V. A., Rykov, A. G., Timofeev, G. A. & Yakovlev, G. N. Investigations of the kinetics of redox reactions of the actinide elements. XX. Kinetics and mechanism of the interaction of americium(III) and (V) with peroxydisulfate ions in nitric acid solution. Sov. Radiochem.13, 851–857 (1971).
Ohyoshi, A., Jyo, A. & Shinohara, T. Kinetics of the reaction of americium(III) with peroxydisulfate. Bull. Chem. Soc. Jpn. 44, 3047–3051 (1971).
Newton, T. W. Kinetics of the Oxidation-Reduction Reactions of Uranium, Neptunium, Plutonium, and Americium in Aqueous Solutions (Los Alamos National Lab. (LANL), Los Alamos, 1975).
Kamoshida, M., Fukasawa, T. & Kawamura, F. Preparation of hexavalent americium in nitric acid solution. J. Nucl. Sci. Technol. 32, 779–786 (1995).
Stokely, J. R. & Moore, F. L. New separation method for americium based on liquid-liquid extraction behavior of americium(V). Anal. Chem. 39, 994–997 (1967).
Mason, G. W., Bollmeier, A. F. Jr & Peppard, D. F. Separation of americium and curium. US Patent 3743696 (1973).
Myasoedov, B. F., Milyukova, M. S. & Litvina, M. N. Oxidation of Am(III) to Am(IV) by a mixture of ammonium persulfate with silver in phosphoric acid solutions. Radiochem. Radioanal. Lett. 25, 33–40 (1976).
Kamoshida, M., Fukasawa, T. & Kawamura, F. Valence control and solvent extraction of americium in the presence of ammonium phosphotungstate. J. Nucl. Sci. Technol. 35, 185–189 (1998).
Kazi, Z. et al. Effective separation of Am(III) and Cm(III) using a DGA resin via the selective oxidation of Am(III) to Am(V). J. Radioanal. Nucl. Chem. 321, 227–233 (2019).
Mason, G. W., Bollmeier, A. F. & Peppard, D. F. Partition of oxidized americium from actinides(III) and lanthanides(III). J. Inorg. Nucl. Chem. 32, 1011–1022 (1970).
Kolthoff, I. M. & Miller, I. K. The chemistry of persulfate. I. The kinetics and mechanism of the decomposition of the persulfate ion in aqueous medium. J. Am. Chem. Soc. 73, 3055–3059 (1951).
Asprey, L. B. & Penneman, R. A. Preparation and properties of aqueous tetravalent americium. Inorg. Chem. 1, 134–136 (1962).
Coleman, J. S. The kinetics of the disproportionation of americium(V). Inorg. Chem. 2, 53–57 (1963).
Cohen, D. Americium(VI) in basic solution. Inorg. Nucl. Chem. Lett. 8, 533–535 (1972).
Tsushima, S., Nagasaki, S. & Suzuki, A. Photochemical oxidation of americium in dilute nitric acid solution with the addition of ozone. Sep. Sci. Technol. 31, 2443–2453 (1996).
Nikonov, M. V., Gogolev, A. V., Tananaev, I. G. & Myasoedov, B. F. Oscillatory reactions Am(VI)⇄ Am(V) under ozonation of Am(OH)3 suspension in bicarbonate solution. Radiochemistry 46, 246–248 (2004).
Felker, L. K., Benker, D. E., Chattin, F. R. & Stacy, R. G. Separation of americium, curium, and plutonium from irradiated targets. Sep. Sci. Technol. 30, 1769–1778 (1995).
Holcomb, H. P. Separation of americium from curium with calcium fluoride. Anal. Chem. 36, 2329–2332 (1964).
Holcomb, H. P. Analytical oxidation of americium with sodium perxenate. Anal. Chem. 37, 415–415 (1965).
Shilov, V. P., Gogolev, A. V. & Fedosseev, A. M. Photochemical oxidation of AmIII in KHCO3 + K2CO3 solutions containing XeO3. Russ. Chem. Bull. Int. Ed. 68, 1458–1459 (2019).
Kulyako, Y. M. et al. Separation of americium and curium in nitric acid solutions via oxidation of Am(III) by bismuthate and perxenate ions. Radiochemistry 62, 581–586 (2020).
Hara, M. The chemistry of americium. I. A study of the preparation of Am(V) and its behavior by means of TTA extraction. Bull. Chem. Soc. Jpn. 43, 89–94 (1970).
Mincher, B. J. Americium Separation from Nuclear Fuel Dissolution Using Higher Oxidation States (Idaho National Lab. (INL), Idaho Falls, 2009).
Mincher, B. J. et al. The solvent extraction of Am(VI) using centrifugal contactors. J. Radioanal. Nucl. Chem. 307, 1833–1836 (2016).
McCann, K., Mincher, B. J., Schmitt, N. C. & Braley, J. C. Hexavalent actinide extraction using N,N-dialkyl amides. Ind. Eng. Chem. Res. 56, 6515–6519 (2017).
Law, J. D., Mincher, B. J., Tillotson, R. D., Schmitt, N. C. & Grimes, T. S. Oxidation and extraction of Am(VI) using a monoamidic extractant in 3D printed centrifugal contactors. J. Radioanal. Nucl. Chem. 318, 35–41 (2018).
Verma, P. K., Bhattacharyya, A. & Mohapatra, P. K. Interlayer confinement mediated oxidation of americium by sodium bismuthate and stability of its higher redox states in acidic solution. Dalton Trans. 53, 15890–15902 (2024).
Labb, S. A., Kmak, K. N., Despotopulos, J. D., Kerlin, W. M. & Sudowe, R. Group hexavalent actinide separation from lanthanides using sodium bismuthate chromatography. J. Chromatogr. A 1736, 465400 (2024).
Hara, M. & Suzuki, S. The chemistry of americium. IV. The stability of Am(V) and Am(VI) in nitric acid solutions and in the solutions containing ozone gas, fluoride, or phosphate ions. Bull. Chem. Soc. Jpn. 52, 1041–1045 (1979).
Rice, N. T. et al. Oxidizing americium(III) with sodium bismuthate in acidic aqueous solutions. Inorg. Chem. 61, 12948–12953 (2022).
Verma, P. K., Bhattacharyya, A., Samanta, S. & Mohapatra, P. K. A highly efficient in situ redox stabilization strategy for Am-Cm separation using AgBiO3. Dalton Trans. 53, 13583–13590 (2024).
Shatokhina, O. B., Alekseeva, D. P., Peretrukhin, V. F. & Krot, N. N. Effects of copper(III) in a periodate complex on hexavalent neptunium and plutonium in an alkaline medium. Radiochemistry 19, 824–828 (1977).
McCann, K., Brigham, D. M., Morrison, S. & Braley, J. C. Hexavalent americium recovery using copper(III) periodate. Inorg. Chem. 55, 11971–11978 (2016).
McCann, K., Sinkov, S. I., Lumetta, G. J. & Shafer, J. C. Organic and aqueous redox speciation of Cu(III) periodate oxidized transuranium actinides. Ind. Eng. Chem. Res. 57, 1277–1283 (2018).
Myasoedov, B. F., Milyukova, M. S., Lebedev, I. A., Litvina, M. N. & Frenkel, V. Y. Behaviour of americium(IV) in phosphoric acid solutions. J. Inorg. Nucl. Chem. 37, 1475–1478 (1975).
Myasoedov, B. F., Milyukova, M. S., Kuzovkina, E. V., Malikov, D. A. & Varezhkina, N. S. Extraction of tetravalent americium. J. Less Common Met. 122, 195–198 (1986).
Donnet, L. et al. Development of the SESAME Process (SCK-CEN, Mol, Belgium, 1998).
Hobart, D. E., Samhoun, K. & Peterson, J. R. Spectroelectrochemical studies of the actinides: stabilization of americium(IV) in aqueous carbonate solution. Radiochim. Acta 31, 139–146 (1982).
Myasoedov, B. F., Lebedev, I. A., Khizhnyak, P. L., Timofeev, G. A. & Frenkel, V. Y. Electrochemical oxidation of americium and californium in carbonate solutions. J. Less Common Met. 122, 189–193 (1986).
Magirius, S., Carnall, W. T. & Kim, J. I. Radiolytic oxidation of Am(III) to Am(V) in NaCl solutions. Radiochim. Acta 38, 29–32 (1985).
Büppelmann, K., Magirius, S., Lierse, C. & Kim, J. I. Radiolytic oxidation of americium(III) to americium(V) and plutonium(IV) to plutonium(VI) in saline solution. J. Less Common Met. 122, 329–336 (1986).
Kynman, A. E. et al. Generation and study of Am(IV) by temperature-controlled electron pulse radiolysis. Dalton Trans. 53, 9262–9266 (2024).
Nikonov, M. V., Shilov, V. P. & Krot, N. N. The influence of ultrasound on redox reactions of americium ions in aqueous solutions. Radiokhimiya 31, 23–26 (1989).
Tsushima, S., Nagasaki, S. & Suzuki, A. Separation of lanthanides and oxidation of americium in nitric acid solution by photolysis. J. Nucl. Sci. Technol. 32, 154–156 (1995).
Carrott, M. J. et al. Oxidation-reduction reactions of simple hydroxamic acids and plutonium(IV) ions in nitric acid. Radiochim. Acta 96, 333–343 (2008).
Bahri, M. A., Ruas, A., Labbé, E. & Moisy, P. Electrochemical characterization of plutonium in n-tributyl phosphate. Dalton Trans. 46, 4943–4949 (2017).
Zhang, Z. C. et al. Complexation-assisted reduction: complexes of glutaroimide-dioxime with tetravalent actinides (Np (IV) and Th (IV)). Dalton Trans. 47, 8134–8141 (2018).
Bourges, J. Y., Guillaume, B., Koehly, G., Hobart, D. E. & Peterson, J. R. Coexistence of americium in four oxidation states in sodium carbonate-sodium bicarbonate medium. Inorg. Chem. 22, 1179–1184 (1983).
Payne, G. F. & Peterson, J. R. Spectroscopic and electrochemical studies of tri- and tetravalent cerium and americium with triphenylarsine oxide in acetonitrile. Radiochim. Acta 39, 155–158 (1986).
Parris, G. E. & Long, G. G. Complexes of tribenzylarsine oxide-II: complexes with lanthanon(III) nitrates. J. Inorg. Nucl. Chem. 32, 1593–1597 (1970).
Cousins, D. R. & Hart, F. A. Lanthanide complexes-IV: complexes of triphenylphosphine oxide with lanthanide and yttrium nitrates. J. Inorg. Nucl. Chem. 29, 1745–1757 (1967).
Cousins, D. R. & Hart, F. A. Lanthanide complexes-V: complexes of yttrium and lanthanide nitrates with triphenylarsine oxide. J. Inorg. Nucl. Chem. 29, 2965–2974 (1967).
Dolbecq, A., Dumas, E., Mayer, C. R. & Mialane, P. Hybrid organic-inorganic polyoxometalate compounds: from structural diversity to applications. Chem. Rev. 110, 6009–6048 (2010).
Miras, H. N., Vila-Nadal, L. & Cronin, L. Polyoxometalate based open-frameworks (POM-OFs). Chem. Soc. Rev. 43, 5679–5699 (2014).
Vila-Nadal, L. & Cronin, L. Design and synthesis of polyoxometalate-framework materials from cluster precursors. Nat. Rev. Mater. 2, 17054 (2017).
Weinstock, I. A., Schreiber, R. E. & Neumann, R. Dioxygen in polyoxometalate mediated reactions. Chem. Rev. 118, 2680–2717 (2018).
Litvina, M. N., Milyukova, M. S. & Myasoedov, B. F. Oxidation of Am(III) and stability of Am(IV) and Am(VI) in mineral acid solutions. J. Radioanal. Nucl. Chem. 121, 355–363 (1988).
Picart, S., Chartier, D., Donnet, L. & Adnet, J. M. Electrochemical Oxidation of Americium in Nitric Medium: Study of Reaction Mechanisms (Commissariat à l´Energie Atomique (CEA), Paris, 2009).
Madic, C., Lecomte, M., Baron, P. & Boullis, B. Separation of long-lived radionuclides from high active nuclear waste. C. R. Phys. 3, 797–811 (2002).
Erin, E. A., Baranov, A. A., Volkov, A. Y., Chistyakov, V. M. & Timofeev, G. A. Electrochemical oxidation of Am(III) ions in HNO3 solutions. Radiochemistry 43, 350–353 (2001).
Erin, E. A., Baranov, A. A., Volkov, A. Y., Chistyakov, V. M. & Timofeev, G. A. Electrochemical oxidation of Am(V) ions in HNO3 solutions. Radiochemistry 46, 33–35 (2004).
Erin, E. A., Baranov, A. A., Volkov, A. Y. & Chistyakov, V. M. Electrochemical oxidation of Am(III) and Am(V) ions in HNO3 solutions containing potassium phosphotungstate K10P2W17O61. Radiochemistry 47, 563–566 (2005).
Zhang, H. L. et al. Ultrafiltration separation of Am(VI)-polyoxometalate from lanthanides. Nature 616, 482–487 (2023).
Asprey, L. B. & Penneman, R. A. First observation of aqueous tetravalent americium. J. Am. Chem. Soc. 83, 2200–2200 (1961).
Moore, F. L. Separation of americium from other elements: application to the purification and radiochemical determination of americium. Anal. Chem. 35, 715–719 (1963).
Keenan, T. K. Lattice constants of some alkali metal actinyl(V) compounds. Inorg. Chem. 4, 1500–1501 (1965).
Sokolova, M. N. et al. Synthesis and structural examination of complexes of Am(IV) and other tetravalent actinides with lacunary heteropolyanion α2-P2W17O6110-. Inorg. Chem. 48, 9185–9190 (2009).
Anan Ev, A. V. S. V. Homogeneous catalysis in actinide chemistry. Radiochemistry 48, 105–118 (2006).
Shannon, R. D. Revised effective ionic-radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 32, 751–767 (1976).
Hall, G. R. & Markin, T. L. The self-reduction of americium(V) and (VI) and the disproportionation of americium(V) in aqueous solution. J. Inorg. Nucl. Chem. 4, 296–303 (1957).
Acknowledgements
C.X. acknowledges the financial support from the National Natural Science Foundation of China (22325603). Z.P.W. acknowledges the financial support from the National Natural Science Foundation of China (22376116) and the Young Elite Scientists Sponsorship Program by CAST (2023QNRC001).
Author information
Authors and Affiliations
Contributions
Y.X.G., Z.P.W., and C.X. conceived the idea. B.L. and L.Z. drafted the manuscript. H.X.H. and Y.S.Y. participated in the discussion. Y.X.G., Z.P.W., and C.X. finalized the manuscript with the input from all the authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work. Peer review reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, B., Zhang, L., Hao, H. et al. Exploiting the coordination chemistry of high-valent americium for actinide/lanthanide separations. Commun Chem 8, 260 (2025). https://doi.org/10.1038/s42004-025-01668-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-025-01668-y