Characterization of Terpene synthase variation in flowers of wild aquilegia species from Northeastern Asia
- PMID: 35039842
- PMCID: PMC8771452
- DOI: 10.1093/hr/uhab020
Characterization of Terpene synthase variation in flowers of wild aquilegia species from Northeastern Asia
Abstract
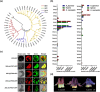
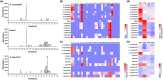
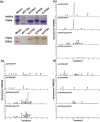
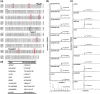
There are several causes for the great diversity in floral terpenes. The terpene products are determined by the catalytic fidelity, efficiency and plasticity of the active sites of terpene synthases (TPSs). However, the molecular mechanism of TPS in catalyzing terpene biosynthesis and its evolutionary fate in wild plant species remain largely unknown. In this study, the functionality of terpene synthases and their natural variants were assessed in two Northeastern Asia endemic columbine species and their natural hybrid. Synoptically, TPS7, TPS8, and TPS9 were highly expressed in these Aquilegia species from the Zuojia population. The in vitro and in vivo enzymatic assays revealed that TPS7 and TPS8 mainly produced (+)-limonene and β-sesquiphellandrene, respectively, whereas TPS9 produced pinene, similar to the major components released from Aquilegia flowers. Multiple sequence alignment of Aquilegia TPS7 and TPS8 in the Zuojia population revealed amino acid polymorphisms. Domain swapping and amino acid substitution assays demonstrated that 413A, 503I and 529D had impacts on TPS7 catalytic activity, whereas 420G, 538F and 545 L affected the ratio of β-sesquiphellandrene to β-bisabolene in TPS8. Moreover, these key polymorphic amino acid residues were found in Aquilegia species from the Changbai Mountain population. Interestingly, amino acid polymorphisms in TPSs were present in individuals with low expression levels, and nonsynonymous mutations could impact the catalytic activity or product specificity of these genes. The results of this study will shed new light on the function and evolution of TPS genes in wild plant species and are beneficial to the modification of plant fragrances.
© The Author(s) 2022. Published by Oxford University Press. All rights reserved.
Figures


References
-
- Degenhardt J, Köllner TG, Gershenzon J. Monoterpene and Sesquiterpene synthases and the origin of Terpene skeletal diversity in plants. Phytochemistry. 2009;70:1621–37. - PubMed
-
- Nagegowda DA. Plant volatile terpenoid metabolism: biosynthetic genes, transcriptional rRegulation and subcellular compartmentation. FEBS Lett. 2010;584:2965–73. - PubMed
LinkOut - more resources
Full Text Sources

