Divergent effects of PERK and IRE1 signaling on cell viability
- PMID: 19137072
- PMCID: PMC2614882
- DOI: 10.1371/journal.pone.0004170
Divergent effects of PERK and IRE1 signaling on cell viability
Abstract
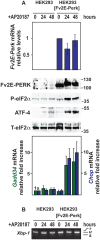
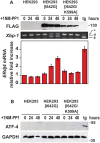
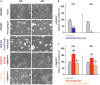
Protein misfolding in the endoplasmic reticulum (ER) activates a set of intracellular signaling pathways, collectively termed the Unfolded Protein Response (UPR). UPR signaling promotes cell survival by reducing misfolded protein levels. If homeostasis cannot be restored, UPR signaling promotes cell death. The molecular basis for the switch between prosurvival and proapoptotic UPR function is poorly understood. The ER-resident proteins, PERK and IRE1, control two key UPR signaling pathways. Protein misfolding concomitantly activates PERK and IRE1 and has clouded insight into their contributions toward life or death cell fates. Here, we employed chemical-genetic strategies to activate individually PERK or IRE1 uncoupled from protein misfolding. We found that sustained PERK signaling impaired cell proliferation and promoted apoptosis. By contrast, equivalent durations of IRE1 signaling enhanced cell proliferation without promoting cell death. These results demonstrate that extended PERK and IRE1 signaling have opposite effects on cell viability. Differential activation of PERK and IRE1 may determine life or death decisions after ER protein misfolding.
Conflict of interest statement
Figures

References
-
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. - PubMed
-
- Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. - PubMed
-
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. - PubMed
-
- Zhou D, Palam LR, Jiang L, Narasimhan J, Staschke KA, et al. Phosphorylation of eIF2 directs ATF5 translational control in response to diverse stress conditions. J Biol Chem. 2008;283:7064–7073. - PubMed
-
- Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

