Molecular basis of halorespiration control by CprK, a CRP-FNR type transcriptional regulator
- PMID: 18717788
- PMCID: PMC2610375
- DOI: 10.1111/j.1365-2958.2008.06399.x
Molecular basis of halorespiration control by CprK, a CRP-FNR type transcriptional regulator
Abstract
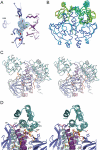
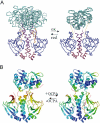
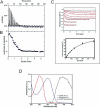
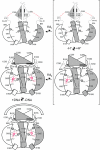
Certain bacteria are able to conserve energy via the reductive dehalogenation of halo-organic compounds in a respiration-type metabolism. The transcriptional regulator CprK from Desulfitobacterium spp. induces expression of halorespiratory genes upon binding of o-chlorophenol ligands and is reversibly inactivated by oxygen through disulphide bond formation. We report crystal structures of D. hafniense CprK in the ligand-free (both oxidation states), ligand-bound (reduced) and DNA-bound states, making it the first member of the widespread CRP-FNR superfamily for which a complete structural description of both redox-dependent and allosteric molecular rearrangements is available. In conjunction with kinetic and thermodynamic ligand binding studies, we provide a model for the allosteric mechanisms underpinning transcriptional control. Amino acids that play a key role in this mechanism are not conserved in functionally distinct CRP-FNR members. This suggests that, despite significant structural homology, distinct allosteric mechanisms are used, enabling this protein family to control a very wide range of processes.
Figures



References
-
- Banerjee R, Ragdale SW. The many faces of vitamin B12: catalysis by cobalamin-dependent enzymes. Annu Rev Biochem. 2003;72:209–247. - PubMed
-
- Benoff B, Yang H, Lawson CL, Parkinson G, Liu J, Blatter E, et al. Structural basis of transcription activation: the CAP-alpha CTD-DNA complex. Science. 2002;297:1562–1566. - PubMed
-
- Borjigin M, Li H, Lanz ND, Kerby RL, Roberts GP, Poulos TL. Structure-based hypothesis on the activation of the CO-sensing transcription factor CooA. Acta Cryst. 2007;D63:282–287. - PubMed
-
- Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Cryst. 1994;D50:760–763. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

