Characterization of mSelB, a novel mammalian elongation factor for selenoprotein translation
- PMID: 10970870
- PMCID: PMC302067
- DOI: 10.1093/emboj/19.17.4796
Characterization of mSelB, a novel mammalian elongation factor for selenoprotein translation
Abstract
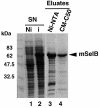
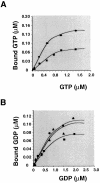
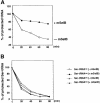
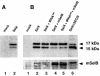
Decoding of UGA selenocysteine codons in eubacteria is mediated by the specialized elongation factor SelB, which conveys the charged tRNA(Sec) to the A site of the ribosome, through binding to the SECIS mRNA hairpin. In an attempt to isolate the eukaryotic homolog of SelB, a database search in this work identified a mouse expressed sequence tag containing the complete cDNA encoding a novel protein of 583 amino acids, which we called mSelB. Several lines of evidence enabled us to establish that mSelB is the bona fide mammalian elongation factor for selenoprotein translation: it binds GTP, recognizes the Sec-tRNA(Sec) in vitro and in vivo, and is required for efficient selenoprotein translation in vivo. In contrast to the eubacterial SelB, the recombinant mSelB alone is unable to bind specifically the eukaryotic SECIS RNA hairpin. However, complementation with HeLa cell extracts led to the formation of a SECIS-dependent complex containing mSelB and at least another factor. Therefore, the role carried out by a single elongation factor in eubacterial selenoprotein translation is devoted to two or more specialized proteins in eukaryotes.
Figures




References
-
- Atkins J.F., Böck,A., Matsufuji,S. and Gesteland,R.F. (1999) Dynamics of the genetic code. In Gesteland,R.F., Cech,T.R. and Atkins,J.F. (eds), The RNA World. 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 637–673.
-
- Berry M.J., Banu,L., Chen,Y.Y., Mandel,S.J., Kieffer,J.D., Harney,J.W. and Larsen,P.R. (1991) Recognition of UGA as a selenocysteine codon in type I deiodinase requires sequences in the 3′ UTR. Nature, 353, 273–276. - PubMed
-
- Buettner C., Harney,J.W. and Berry,M.J. (1999) The Caenorhabditis elegans homologue of thioredoxin reductase contains a SECIS element that differs from mammalian SECIS elements but directs selenocysteine incorporation. J. Biol. Chem., 274, 21598–21602. - PubMed
-
- Burk R.F. and Hill,K.E. (1999) Orphan selenoproteins. BioEssays, 21, 231–237. - PubMed
-
- Commans S. and Böck,A. (1999) Selenocysteine inserting tRNAs: an overview. FEMS Microbiol. Rev., 23, 335–351. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

