Abstract
Background
Bone nonunion or delayed union is a serious complication in diabetic patients with fractures, urgently requiring novel therapeutic strategies. Exosomes derived from stromal cells are naturally occurring nanoparticles carrying bioactive molecules that mediate intercellular communication and play crucial roles in diabetic fracture repair. Importantly, mechanical stimuli can modulate the cargo composition of exosomes, influencing bone healing outcomes. Here, we investigate for the first time whether exosomes derived from mechanically stretched adipose-derived stromal cells (MS-ADSC-Exos) enhance fracture healing in a type 2 diabetes mellitus (T2DM) nonunion model, and elucidate their underlying mechanisms.
Methods
Exosomes secreted by ADSCs subjected to different magnitudes of cyclic mechanical stretch (0%, 6%, 18%; designated NMS, LMS, and HMS-ADSC-Exos) were applied to rat bone marrow mesenchymal stromal cells (BMSCs) and human umbilical vein endothelial cells (HUVECs) in vitro. Osteogenic differentiation, proliferation, migration, and angiogenesis were evaluated by Alizarin Red S and ALP staining, tube formation, scratch, and migration assays, respectively. Western blotting and immunofluorescence assessed osteogenic marker expression. In vivo, MS-ADSC-Exos or PBS were locally injected into the fracture sites of diabetic rat femoral nonunion models for 3 consecutive days post-operation. Bone regeneration was evaluated by micro-CT and histological analyses at 4 weeks. miRNA profiles of MS-ADSC-Exos were characterized by RNA sequencing, bioinformatics, and qRT-PCR. Functional roles of miR-877 were further validated via mimic and inhibitor transfection assays.
Results
In this study, it is shown that exosomes secreted from ADSCs induced via lower mechanical stretch can enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion with T2DM. Our results suggested miR-877 was significantly upregulated in LMS-ADSC-Exos, and can be transferred into BMSCs and HUVECs, which promotes osteogenesis and angiogenesis in diabetic conditions.
Conclusions
This study reveals a novel mechanobiological mechanism whereby mechanical stretch modulates exosomal miRNA content to potentiate fracture repair. Transplantation of LMS-ADSC-Exos accelerates bone regeneration via miR-877-mediated osteogenic and angiogenic pathways. These findings highlight the therapeutic potential of mechanically stimulated ADSC-derived exosomes as natural bioactive nanotherapeutics for diabetic fracture nonunion.
Similar content being viewed by others
Introduction
Bone nonunion or delayed union is a serious complication of fractures [1, 2]. Non-union represents a chronic medical condition that affects function, and potentially impacts the individual’s psychosocial and economic well-being, causing an exaggerated economic and mental burden to patients [1, 3]. Diabetes substantially alters bone metabolism, bone structure and soft tissue healing, increases fracture and infection risk and impairs fracture-healing, leading to exacerbated nonunion and delayed fracture-healing [4, 5].
ADSCs are multipotent progenitor/stromal cells in adipose tissue. ADSCs have potent multi-directional differentiation potential, including adipogenic, osteogenic and chondrogenic differentiation, which play a crucial role in maintenance and repair of tissues throughout the life time [6, 7]. Adipocytes are powerful endocrine cells, secreting multiple bioactive peptides and extracellular vesicles [8, 9]. In addition to its presence in subcutaneous, visceral and bone marrow depots, adipose tissue is distributed ubiquitously within other organs and tissues. As a primary source of ADSCs, adipose tissue derived from subcutaneous tissue is abundant and easy to obtain. ADSCs are abundant in source, minimally invasive and easy to obtain. Therefore, ADSCs are widely applied in regenerative medicine and plastic surgery [10, 11]. However, applying adipocytes or adipose-derived stromal cells transplantation directly in the clinic is frustrated by underlying immunosuppression, cell dedifferentiation and tumor formation. But exosomes derived from ADSC can avoid those side effects and risk perfectly [12]. Recently, exosomes of ADSCs have emerged and are replacing ADSCs in research such as regenerative medicine and tissue repair and reconstruction [13, 14]. Exosomes are small cell-secreted vesicles (30–150 nm) containing and transmitting sophisticated RNA and protein cargos in a cell-to-cell manner, which are the bases of highly efficient intercellular communication [15, 16]. It has been confirmed that stromal cell exosomes can effectively promote osteogenic differentiation of MSC and bone healing [17, 18]. Therefore, the use of stromal cell exosomes to interfere bone healing process has great potential.
Mechanical stretch plays a vital role in processes such as MSC differentiation, the formation of blood vessels, which are critical for bone fracture healing. Cells sense, translate, and transmit mechanical signals from their periphery to the nucleus and induce changes in gene expression, and further affect the biological behavior of the recipient cells involved in bone healing, resulting in non-union or delayed healing [19, 20]. Exosomes are able to be derived from various cell types, and their characterization is similar to derived cells. Therefore, we aim to observe the effect of ADSC-derived exosomes under different magnitudes/intensities of mechanical stress (cyclic tensile stress) on osteogenic differentiation of BMSC in vivo and in vitro, and further demonstrate that stress-induced ADSCs-derived exosomes promote fracture healing in rats via up-regulating microRNA-877.
Materials and methods
Establishment of T2DM rats fracture model and rats treatments
The work has been reported in line with the ARRIVE guidelines 2.0.
After adapting to feeding for three days, the Sprague Dawley (SD) rats were weighed and classified by cage (3 rats/cage), and 60 male SD rats of 3 weeks old were randomly distributed into 2 groups. Many studies have shown the procedures of establishing high-fat fed (HFD) and streptozotocin (STZ)-induced diabetic rat model which are a model of T2DM. According to the procedures of induction of the T2DM rat model in the existing study, after 3 weeks of feeding HFD (containing 60% fat), rats in the T2DM group were injected with STZ (40 mg/kg in citrate buffer). The control group fed with a normal pelleted diet received an equal volume of citrate buffer [21, 22].
The blood glucose (BG) levels were evaluated consecutively, and blood samples were collected from the tail vein. According to the procedures, rats with randomly blood glucose (RBG) samples > 16.7 mmol/L more than three times were identified to have T2DM after 7 weeks. During diabetes induction, animals were given free access to their original diets (received the high-fat or control diet and water ad libitum) for 12 weeks.
To assess the T2DM model, we measured the metabolic index, including body weight, food intake, water consumption and volume of excreted urine at several time points, including before being fed the HFD and 12 weeks after STZ injection. In addition, RBG was observed at several special time points, which included before fed HFD, before STZ injection, one week after STZ injection, 6 weeks after STZ injection and 12 weeks after STZ injection. At the end of the observation, IPGTT and ITT were evaluated. In detail, to execute IPGTT, animals were fasted for 12 h and injected with 1.5 g/kg glucose. BG was measured at 0, 30, 60 and 120 min after glucose injection. While ITT was carried out by injecting the rats with 0.75 IU/kg insulin, and then BG was obtained at 0, 30, 60 and 120 min after insulin administration. Animals with an RBG below 10 mmol/L at any time points were regarded as nondiabetic, and those with an RBG between 10 and 16.7 mmol/L were excluded. At 12 weeks after STZ injection, rats remaining in the T2DM group were evaluated as diabetic rats in a model of T2DM [23, 24].
After 15 weeks of treatment, all the successfully induced T2DM rats received general anesthesia with pentobarbital sodium intraperitoneal injection before surgery. A lateral incision was made along the proximal femur, followed by longitudinal dissection of the skin, subcutaneous tissue, and muscle along the femoral axis. The surrounding soft tissues were gently separated to expose the femur. A transverse osteotomy of the mid-diaphysis of the femur was performed by an oscillating mini-saw to establish a transverse femur shaft fracture model. The knee was bent 90 degrees, the lateral patellar incision was made, and the Kirschner needle was inserted femur intramedullary through the femoral intercondylar groove. The tip of the Kirschner needle was inserted through the top of the femoral greater trochanter to stabilize the fracture. Finally, all the incisions were closed using a 4-0 nylon suture. All the rats were kept in individual cages. Unprotected weight bearing was allowed immediately after the operation. After surgery, all the animals were given food and water ad libitum. At the end of the experiments, a sodium pentobarbital solution was administered intraperitoneally at a dose of 200 mg/kg. Following administration, animals were carefully monitored until the complete cessation of respiration and heartbeat, and a secondary physical method was applied when necessary to confirm death. All procedures were performed by personnel trained in rodent euthanasia.
Based on X-ray examinations, the fracture sites in the rats were located and marked on their skin. Then, 600 µL of ADSC-exosomes induced by mechanical stretch at a concentration of 200 µg/mL, as well as an equal volume of PBS, were locally injected into the fracture site every three days after surgery, with 200 µL administered each time. Finally, X-ray examination, micro-CT examination, histochemistry analysis, and Western blot analysis of the fractured femurs were performed 28 days after the operation.
Cells obtaining and culture
To obtain ADSCs, the subcutaneous fat in the groin of 3 weeks old male rats was cut into pieces as small as possible and centrifuged at 1500 rpm for 10 min to extract the sediment and glue enzyme was added in to digest at 37 °C for 40 min, then added the medium to terminate the reaction, and the mixture was filtered with a 70 μm filter. After centrifugating at 1500 rpm for 8 min, the cells were suspended in the medium and cultured in vitro for about 8 days. ADSCs (CD44+ CD90+ CD34-CD45-) were collected and certified by flow cytometry and the activity of ADSCs was determined by Calcein Acetoxymethyl Ester (Calcein AM) (Beyotime Biotechnology, Shanghai, China) staining assay.
Bone marrow cells were extracted from the femur and tibia of 3-week-old male rats, then isolated and cultured for 3 generations. BMSCs (CD44+ , CD34-) were identified by flow cytometry, and the cellular bioactivity of BMSCs was determined by Calcein AM staining assay.
The HUVECs (PUMC-HUVEC-T1; Cat NO: CL-0675) used in our experiments were originally purchased from a commercial vendor (Wuhan Pricella Biotechnology Co., Ltd.). To mimic the true condition and environment of Type 2 Diabetes Mellitus. All cells used in cellular function experiments were cultured with a complete culture medium consisting of high-concentration glucose (30 mM).
Tensile strain treatments of cells
Cell’s mechanical stimulation device has become a well-established method to apply mechanical strain to cultured cells; numerous similar devices for cell stimulation have been developed. In this study, external tensile strain was applied to ADSCs using a cell tension culture system. The special elastic membrane or well of the cell tension culture system was precoated with Matrigel (Corning, Bedford, MA, USA), incubated overnight, and kept moist with PBS for later use. ADSCs were cultured on the coated membrane or well. Cyclic tensile strain was applied using a uniaxial elongation model either 24 h post-seeding or upon reaching 90–100% confluence to simulate mechanical stimulation. In the selected uniaxial elongation model, cyclic sinusoidal tensile strain at a fixed frequency was applied to the cells according to a predefined protocol. All the ADSCs were divided into 3 groups, in which ADSCs were given of 1.0 Hz 6% (lower magnitude mechanical stretch ADSCs, LMS), 1.0 Hz 18% (higher magnitude mechanical stretch ADSCs, HMS) tensile strain, 2 h per day separately, and a total of 3 days. In the non-stress group (non-mechanical stretch ADSCs, NMS), 0% tensile strain was applied to ADSCs for 3 days in succession [25,26,27].
Isolation and identification of ADSC exosomes
After exposure to the corresponding tensile strain, ADSCs were washed three times with PBS and then cultured in exosome-free, FBS-free basal medium for an additional 24 h. Exosomes were isolated using the technique of differential centrifugation. In detail, the supernatant was collected and centrifuged at 1000×g at 4 °C for 10 min, 2000×g at 4 °C for 10 min, then centrifuged at 10000×g at 4 °C for 30 min, and centrifuged at 140000×g at 4 °C for 90 min in turn using a Beckman Coulter ultracentrifuge (Beckman Coulter, USA). Discard supernatant PBS washing sample, 140000×g centrifuge for 90 min at 4 °C. The precipitated exosomes were collected and re-suspended in 0.5 mL PBS and conserved at − 80 °C [28]. Then the volume of exosomes was concentrated to 200 μL. The morphology of exosomes was observed by transmission electron microscope (TEM; HITACHI, HT7700, JAPAN) and the diameter distribution was analyzed by Nanoparticles Tracking Analysis (NTA; ZetaView, Particle Metrix, Meerbusch, Germany). Western blot analysis identified its specific biomarkers (CD9, CD63, TSG101).
Flow cytometry (FCM) assay
ADSCs were analyzed by flow cytometry. ADSCs were detected with specific biomarker antibodies CD44 (Affinity), CD90 (Abcam), CD34, and CD45 (Abcam). BMSCs were certified by specific biomarkers CD44 (Affinity) and CD34 (Abcam). Results were analyzed using Flowjo software (version 10.0; BD Biosciences).
Exosome uptake assay
Based mainly on the manufacturer’s standard procedure, PKH26 was used as a dye to label the exosomes in the exosome uptake assay. In short, exosomes were obtained with differential centrifugation (140,000×g, 20 min, 4 °C) dyed with PKH26 (mixed solution was incubated for 20 min at room temperature). While BMSCs were seeded onto a 35 mm confocal dish at the proper density and labeled with DAPI dye. Then, exosomes labeled with PKH26 were mixed and co-cultured with BMSCs labeled with DAPI for 12 h and finally observed via a confocal laser scanning microscopy (CLSM, Leica Microsystems, Germany).
ALP activity and mineralization assessment of osteogenic differentiation
Following three passages of culture, BMSCs were seeded into 6-well plates (2 × 105 cells per well) that had been pre-coated with a 0.1% gelatin solution. The plates were then incubated for 14 days using a specific osteogenic induction medium (Cyagen Biosciences). To evaluate the effect of ADSCs-Exos on osteogenic differentiation, 200 µL of ADSCs-Exos (LMS-Exos, NMS-Exos, HMS-Exos derived from ADSCs) with a concentration of 200 µg/mL and equal volumes of PBS were added into every well severally with the osteogenic induction medium, and the medium was refreshed every three days. To evaluate the level of osteogenic differentiation, the cells were stained with alizarin red staining (ARS) dye and alkaline phosphatase (ALP) staining, and were collected for Western Blotting analysis on day 14. In detail, BMSCs were osteogenic induced for 14 days with different treatments, then cells were washed two times via PBS, then fixed with 4% paraformaldehyde for 30 min at room temperature prepare for ALP and ARS staining. A BCIP/NBT ALP kit (Beyotime, China) was used for ALP staining. After the stained cells were washed using PBS three times, the BCIP/NBT substrate was utilized to stain osteogenic-induced BMSCs. The results were observed and imaged via optical microscopy and then calculated and evaluated by the Image J software processing system (NIH, USA), and GraphPad Prism 10.0 (GraphPad Software, CA, USA) was used for the data analysis.
Tube formation assay of ADSC-Exos
HUVECs were cultured in Matrigel (Corning, USA) precoated 24-well plates (1 × 105 cells per well). 200 µL of ADSCs-Exos (LMS-Exos, NMS-Exos, HMS-Exos derived from ADSCs) with a concentration of 200 µg/mL and equal volumes of PBS were added into every well with the medium, respectively. 6 h later, tube formation was observed with a fluorescence microscope. The Image J software processing system (NIH, USA) and GraphPad Prism 10.0 (GraphPad Software, CA, USA) were used for quantification and data analysis.
Scratch test and migration test
HUVECs were cultured in 6-well plates (2 × 105 cells per well). 200 µL of ADSCs-Exos (LMS-Exos, NMS-Exos, HMS-Exos derived from ADSCs) with a concentration of 200 µg/mL and equal volumes of PBS were added into every well with the medium without serum. 24 h later when cultured HUVEC cells reached 100% confluence, a straight scratch was made with a 200 µL pipette in every well. 0 h, scratched wounds were observed with a fluorescence microscope and recorded. 12 h later, Scratch wound healing results were observed with a fluorescence microscope and photos were taken. Before observation, all samples were stained with calcein AM dye (Beyotime Biotechnology, Shanghai, China). The Image J software processing system (NIH, USA) and GraphPad Prism 10.0 (GraphPad Software, CA, USA) were used for quantification and data analysis.
Transwell assay
HUVECs treated with 200 µL of ADSCs-Exos (LMS-Exos, NMS-Exos, HMS-Exos derived from ADSCs) with a concentration of 200 µg/mL and equal volumes of PBS were seeded into 24-well transwell of 8 µm pore diameter cell culture plate (Corning, USA). 12 h later, HUVECs were stained with crystal violet for 20 min, and then observed by an optical microscope. The Image J software processing system (NIH, USA) and GraphPad Prism 10.0 (GraphPad Software, CA, USA) were used for quantification and the data analysis.
Cell alive/dead assays
All cells, including ADSCs, HUVECs, and BMSCs, were stained before use via calcein AM and Propidium iodide (PI) dye (Beyotime Biotechnology, Shanghai, China) to evaluate cell vitality.
Western blot analysis
Western blotting was performed following previously described protocols. First, after the concentrations of protein were measured via BCA (Aspen), the protein was separated into equal amounts via SDS-PAGE (Beyotime, China), transferred into the PVDF membrane (Millipore, Burlington, MA, USA) and then incubated with 5% bovine serum albumin for 1 h at 25 °C. Next, the membranes were incubated overnight with primary antibodies specific for CD9 (Abcam), CD63 (Abcam), TSG101 (Abcam), Runx2 (Abcam), OCN (Santa), and GAPDH (Abcam). HRP-labeled secondary antibody (Abcam, USA) was added, and then, the membrane was visualized using a T5200 Multi Chemiluminescence Detection System (Tanon, China) as recommended of the manufacturer. The membranes were incubated with Immobilon ECL reagent (Thermo Fisher Scientific, Waltham, MA, USA), and the bands were quantified via Image J software (NIH, USA). GAPDH protein level was used as an internal control for MACF1. The Image J software processing system (NIH, USA) and GraphPad Prism 10.0 (GraphPad Software, CA, USA) were used for quantification of protein level and the data analysis. The significance level was set to a 95% confidence interval, and statistical significance was declared as * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 and ns p > 0.05.
Micro-CT analysis
The animals were euthanized 4 weeks postoperatively, the internal fixations were removed and their femurs were fixed in 4% paraformaldehyde for 24 h at 4 °C. Then, tissue specimens were scanned via a micro-CT system (SkyScan1276, Bruker, Belgium) at a resolution of 9.054604 μm with 85 kV and 200 µA. After scanning, 3D structures of femurs were performed (Reconstruction was accomplished by NRecon (version 1.7.4.2)), and the new bone volume/total volume (BV/TV) were calculated to assess bone regeneration in the fracture site to assess the morphology of the reconstructed femurs (3D and 2D analysis were performed using software CT Analyser (version 1.20.3.0)).
qRT‑PCR analysis
Total RNA was isolated from exosomes using TRIZOL Extraction Reagent (G3013, Servicebio). The cDNA was reverse transcribed using RevertAid First Strand cDNA Synthesis Kit (Invitrogen, CA, USA) following the manufacturer’s instructions. And the qRT-PCR for mRNAs was performed on a StepOne™ Real-Time PCR System (Life technologies) using EnTurboSYBR Green PCR SuperMix (EQ001, ELK Biotechnology) or HieffTM qPCR SYBR™ Green Master Mix (No Rox Plus) (11201ES, Shanghai Yeasen BioTechnologies). The relative expression levels of mRNA or miRNA were normalized to those of GAPDH or U6 and evaluated using the 2−ΔΔCT method. The primers used for qRT-PCR are listed in Table 1.
Transfection
Following manufacturer’s protocols, miR-877 mimics or inhibitors and their NCs (Generalbiol, HeFei, China) were transfected into BMSCs and HUVECs to evaluate miR-877 function. After 48 h of transfection, the expression level of miR-877 was measured by qRT-PCR.
RNA sequencing and bioinformatics analysis
All the samples were processed as description previously. All the experiment procedures were according manufacture’s protocols and recommendations. Briefly, Total RNA was extracted from exosomes using TRIzol reagent (Invitrogen, CA, USA) according to the manufacturer’s protocol. Purity, concentration and integrity of RNA sample were examined by NanoDrop, Qubit 2.0, Agilent 2100, etc. RNA concentration and purity was measured using NanoDrop 2000 (Thermo Fisher Scientific, Wilmington, DE). RNA integrity was assessed using the RNA Nano 6000 Assay Kit of the Agilent Bioanalyzer 2100 system (Agilent Technologies, CA, USA). Only RNA with good quality could move on to following procedures. Total RNA from each sample was used to prepare miRNA library using NEB Next Ultra small RNA Sample Library Prep Kit (NEB, Boston, MA, USA) according to the Illumina small RNA sample preparation protocol. Sequencing was then performed on the Illumina novaseq6000 platform (Illumina, San Diego, CA). A total amount of 1.5 μg RNA per sample was used as input material for the RNA sample preparations. Briefly, first of all, ligated the 3′SR Adaptor. Mixed 3′SR Adaptor for Illumina, RNA and Nuclease-Free Water, after mixture system incubation for 2 min at 70 °C in a preheated thermal cycler, the Tube was transfer to ice. Then, add 3′ Ligation Reaction Buffer (2X) and 3′Ligation Enzyme Mix ligate the 3′SR Adaptor. Incubated for 1 h at 25 °C in a thermal cycler. To prevent adaptor-dimer formation, the SR RT Primer hybridizes to the excess of 3′SR Adaptor (that remains free after the 3′ligation reaction) and transforms the single stranded DNA adaptor into a double-stranded DNA molecule. And dsDNAs are not substrates for ligation mediated. The second, ligated the 5′SR Adaptor. Then, reverse transcription synthetic first chain. Last, PCR amplification and Size Selection. PAGE gel was used for electrophoresis fragment screening purposes, rubber cutting recycling as the pieces to get small RNA libraries. At last, PCR products were purified (AMPure XP system) and library quality was assessed on the Agilent Bioanalyzer 2100 system. The clustering of the index-coded samples was performed on a cBot Cluster Generation System using TruSeq PE Cluster Kit v4-cBot-HS (Illumia) according to the manufacturer’s instructions. After cluster generation, the library preparations were sequenced on an Illumina Hiseq 2500 platform and paired-end reads were generated. Raw data (raw reads) of fastq format were firstly processed through in-house perl scripts. In this step, clean data (clean reads) were obtained by removing reads containing adapter, ploy-N and low-quality reads from raw data. And reads were trimmed and cleaned by removing the sequences smaller than 18 nt or longer than 30 nt. Differential expression analysis of two groups was performed using the DESeq R package (1.10.1). DESeq provide statistical routines for determining differential expression in digital miRNA expression data using a model based on the negative binomial distribution. The resulting P values were adjusted using the Benjamini and Hochberg’s approach for controlling the false discovery rate. miRNA with an adjusted p < 0.05 found by DESeq were assigned as differentially expressed. Target gene function was annotated based on the following databases:Nr (NCBI non-redundant protein sequences); Nt (NCBI non-redundant nucleotide sequences); Pfam (Protein family); KOG/COG (Clusters of Orthologous Groups of proteins); Swiss-Prot (A manually annotated and reviewed protein sequence database); KO (KEGG Ortholog database); GO (Gene Ontology).
Histological and immunofluorescence analysis
The collected femurs of rats from different groups were fixed in 4% paraformaldehyde solution for 48 h, decalcified with 20% EDTA at 25 °C for 28 days, embedded in paraffin, and sectioned along the longitudinal axis. Sections from the fracture region were stained with hematoxylin and eosin (H&E), safranin O-fast green, Masson, RUNX2, OCN for immunohistochemical analysis. Immunofluorescence staining with α-SMA, CD31, RUNX2, OCN for immunofluorescence analysis. The sections were imaged and observed by a microscope.
Statistical analysis
All the experiments were performed at least three replicates per group. Values were presented as mean ± SD, and were analyzed with GraphPad Prism 10.0 (Graph-Pad Software, CA, USA). Variances between groups were assessed by the two-sided Student’s t-test (for two-group comparisons) or the one-way analysis of variance (ANOVA) with Tukey’s test (for more than two-group comparisons). And statistical significance was declared as* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001; ns p > 0.05, not significant.
Results
Characterization and identification of ADSC-exos
ADSCs were harvested from the subcutaneous fat in the groin of rats and cultured in vitro for about 8 days. ADSCs were succeed obtained, then ADSCs were certificated by flow cytometry specific identifying special biomarkers (CD44 + , CD90 + , CD34-, CD45-), and the activity of ADSCs was detected by Calcein Acetoxymethyl Ester (Calcein AM) staining assay (Fig. 1A). BMSCs were extracted from the femur and tibia of 3-week-old male rats, then isolated and cultured for 3 generations. BMSCs (CD44 + , CD34-) were identified by flow cytometry and the activity of BMSCs was determined by Calcein Acetoxymethyl Ester (Calcein AM) staining assay (Fig. 1B).
Identification of ADSCs and ADSC-derived exosomes in vitro. A Morphology of ADSCs. B ADSC surface biomarkers (positive for CD44 and CD90, and negative for CD34 and CD45) were certified via flow cytometry. C The morphology of ADSC-derived exosomes was certified via transmission electron microscope (TEM) and analyzed by Nanoparticles Tracking Analysis (NTA) of diameter distribution. D Western blot analysis identified surface markers (CD9, CD63, TSG101) of mechanical stretch-induced exosomes, which were derived from ADSC (Full-length blots/gels are presented in Supplementary Fig. 1). E The internalization of ADSC exosomes by BMSCs was observed via a confocal laser scanning microscopy (CLSM) (ADSC exosomes were co-cultured with BMSCs, Cytoskeleton and Nucleus of BMSCs were stained by Phalloidin and DAPI separately. Exosomes were labeled with red fluorescent dye PKH26)
To certify the exosomes derived from ADSCs, Western blotting, TEM and NTA of diameter distribution were performed. TEM analysis showed that the morphology of the ADSC-Exos was cup-shaped (Fig. 1C). Western blot analysis identified its specific markers (CD9, CD63, TSG101) (Fig. 1D). The particle sizes of ADSC-Exos were evaluated using NTA, and the results showed that their size distribution ranged from 30 to 200 nm, the average diameter were approximately 120 nm. Through fluorescence microscopy (ADSC exosomes were co-cultured with BMSCs, and exosomes were labeled with red fluorescent dye PKH26), the internalization of ADSC exosomes by BMSCs was successfully observed via a confocal fluorescence microscope (Fig. 1E).
Cyclic mechanical stretch-induced ADSCs-exosomes promote osteogenic differentiation of BMSC in vitro
The effects on osteogenesis were evaluated after BMSCs were treated with exosomes derived from ADSCs induced by cyclic mechanical stretch. Firstly, we identified that LMS-Exos significantly promoted BMSC osteogenesis differentiation, but HMS-Exos significantly inhibited osteogenesis of BMSC, when compared with NMS-Exos and PBS group treated BMSC via ARS and ALP staining assays (Fig. 2A, B). Then we investigated osteogenic differentiation of BMSC co-cultured with MS-Exos. RUNX-2, OCN are classic molecular biomarkers of osteogenic differentiation. Consistent with results of ARS and ALP staining assays, when compared to the PBS group, Western blotting and immunofluorescence staining also showed the protein expression levels of RUNX-2, OCN upregulate significantly in LMS-Exos group, and downregulate significantly in HMS-Exos group (Fig. 2C–I). Therefore, we further certified LMS-Exos can accelerate osteogenesis, and HMS-Exos impede osteogenesis in vitro.
Cyclic mechanical stretch induced ADSCs-Exos promote osteogenesis differentiation of BMSCs in vitro. A, B Alizarin red staining and ALP staining of PBS-, NMS-Exos-, HMS-Exos-, LMS-Exos-treated BMSCs after 14-day osteogenic induction. C, D Semi-quantification of Alizarin red staining and ALP staining results (Scale bars, 200 µm). E–H Western blotting of RUNX2 and OCN in PBS-, NMS-Exos-, HMS-Exos-, LMS-Exos-treated BMSCs and quantitative analysis (Full-length blots/gels are presented in Supplementary Fig. 2). I Immunofluorescent staining of RUNX2 and OCN of PBS-, NMS-Exos-, HMS-Exos-, LMS-Exos-treated BMSCs after 14-day osteogenic induction (Scale bars, 50 µm). All experiments were repeated three times. Data are presented as the mean ± SD. Statistical significance was determined by two-sided Student’s t-test, or one-way ANOVA with Tukey’s test (* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001; ns p > 0.05, not significant)
Cyclic mechanical stretch-induced ADSCs-exosomes promote angiogenesis in vitro
To demonstrate the angiogenic effects of Cyclic mechanical stretch induced ADSCs-Exosomes in vitro, PBS, NMS-Exos, HMS-Exos, LMS-Exos, and HUVECs were administered to accomplish Scratch Wound Healing Assay, Transwell Assay, and Tube Formation Assay (Fig. 3A–C). In addition, Western Blotting was performed to evaluate angiogenesis hallmarks VEGF and CD31 expression level in osteogenically induced BMSCs treated with PBS, NMS-Exos, HMS-Exos, and LMS-Exos (Fig. 3G, H). The results of the Scratch Wound Healing assay, Transwell Assay, and Tube Formation Assay revealed that when compared with PBS (negative control), NMS-Exos, LMS-Exos showed optimal effect in strengthening angiogenesis. And HMS-Exos showed an impaired angiogenic effort. Besides, the Western Blotting assay exhibited similar results. Those results confirmed that optimal cyclic mechanical stretch induced ADSCs-exosomes firmly facilitate angiogenesis in vitro.
Cyclic mechanical stretch-induced ADSCs-exosomes promote angiogenesis capability in vitro. A, D Cyclic mechanical stretch induced ADSCs-exosomes enhances the migratory activity of HUVECs at 24 h by scratch wound assay and quantitative analysis of the wound healing rate. B, E Transwell assay and quantitative analysis relative migration rate of the HUVECs. C, F Tube formation of HUVECs and quantitative analysis of the average tube length. G–J Western blotting assay and statistical analysis of the protein expression of VEGF, CD31 of PBS-, NMS-Exos-, HMS-Exos-, LMS-Exos-treated BMSCs after 7-day osteogenic induction (Full-length blots/gels are presented in Supplementary Fig. 3). Scale bars, 200 µm. and all experiments were repeated three times (* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001; ns p > 0.05, not significant)
Cyclic mechanical stretch induced ADSCs-exosomes promote osteogenesis differentiation and bone healing of BMSC in non-union rats fracture model
To further determine the therapeutic effects of the local injection of cyclic mechanical stretch-induced ADSCs-exosomes on non-union rats. We successfully established a transverse femur shaft fracture non-union diabetic rat model.
The femurs of diabetic rats under different treatments were harvested and radiologic photographed on day 28 after surgery (Fig. 4A). X-ray, Micro-CT examination, and parameters of morphology (bone volume/total volume (BV/TV)) revealed that fracture healing accelerated in rats treated by exosomes derived from moderate intensity mechanical stretch-induced ADSCs. Compared with higher intensity stretch-induced ADSC-exosomes treated rats and non-stretched treated rats, lower stretch-induced ADSC-exosomes treated rats exhibited the smallest fracture gap and larger callus volume which indicates the fastest speed of bone regeneration and fracture healing. But high-intensity stretch group rats showed the poorest result in bone regeneration and fracture healing, which indicates high-strength cyclic mechanical stretch exosomes impair bone regeneration and fracture healing (Fig. 4B, C).
Cyclic mechanical stretch-induced ADSCs-exosomes promote osteogenic differentiation of BMSC in Non-union Rats Fracture Model. A Schematic illustration of the local delivery of cyclic mechanical stretch-induced ADSCs-exosomes design. B Micro-CT scans of femurs, which PBS, NMS-Exos-, HMS-Exos-, LMS-Exos- pretreated via fracture region local injection in the fourth week postoperatively (n = 6 rats per group). C Immunohistochemical staining (RUNX2, OCN) in fracture regions from femurs of different groups (PBS, NMS-Exos-, HMS-Exos-, LMS-Exos- pretreated) in the fourth week postoperatively (n = 6 rats per group). D Statistical analysis of BV/TV in each group (n = 4 rats per group). E Immunofluorescence staining with α-SMA, CD31 in fracture regions from femurs of different groups (PBS, NMS-Exos-, HMS-Exos-, LMS-Exos- pretreated) in the fourth week postoperatively (n = 6 rats per group). Data are represented as mean ± SD. Statistical significance was determined by one-way ANOVA with Tukey’s test (* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001; ns p > 0.05, not significant)
Histological analysis results of bone tissue from the fracture region in different magnitude mechanical stretch-induced exosomes-treated femurs were aligned with X-ray and micro-CT examination results in each group (Immunohistochemical staining (RUNX2, OCN), Immunofluorescence staining (α-SMA, CD31)) (Fig. 4D, E).
MiR-877 expression was upregulated in cyclic mechanical stretch-induced ADSCs-exosomes
Because exosomal miRNAs play an important role in bone repair and regeneration, we further identified the differentially expressed miRNAs in exosomes derived from different magnitude mechanical stretch-induced ADSCs. The mRNA expression levels of miRNAs in LMS-ADSC-Exos and NMS-ADSC-Exos were analyzed by miRNA sequencing. And as shown in Fig. 5A, B, C compared to NMS-ADSC-Exos groups, the heat map and Volcano plot analysis displayed that a number of miRNAs were significantly upregulated in LMS-ADSC-Exos.
miR-877 was drastically increased in LMS-ADCS-Exos and it can be transferred to BMSCs and HUVECs via exosomes. A Hot map showed most significantly upregulated and downregulated proteins (LMS-ADSC-Exos vs NMS-ADSC-Exos). B Volcano plot of upregulated and downregulated expressions of miRNAs in LMS-ADSC-Exos compared to those in NMS-ADSC-Exos (|logFC|> 1.3, P < 0.05). C KEEG network based on differentially expressed proteins associated with the significant pathways using the STRING database. D Comparison of the top six elevated miRNAs (miR-145-3p, miR-30c-1-3p, miR-877, miR-450a-3p, miR-33-3p and miR-200b-3p) between LMS-ADSC-Exos and NMS-ADSC-Exos using qRT-PCR analyses. E, F Recipient cells (BMSCs) were co-cultured with exosomes induced by mechanical stretch (NMS-ADSC-Exos, LMS-ADSC-Exos), and miR-877 effectively expressed in recipient cells (BMSCs). Comparison of miR-877 levels among groups following BMSCs treated with PBS, NMS-ADSC-Exos or LMS-ADSC-Exos were determined with qRT-PCR assay, respectively. G, H Recipient cells (HUVECs) were co-cultured with exosomes induced by mechanical stretch (NMS-ADSC-Exos, LMS-ADSC-Exos), and miR-877 effectively expressed in recipient cells (HUVECs). Comparison of miR-877 levels among groups following HUVECs treated with PBS, NMS-ADSC-Exos or LMS-ADSC-Exos were determined with qRT-PCR assay, respectively. Data are presented as the mean ± SD. (* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001; ns p > 0.05, not significant)
Furthermore, qRT-PCR was performed to evaluate miRNAs in ADSC-MS-Exos. The results certified miR-877 possessed the largest extent of expression upregulating among all six upregulating miRNAs (Fig. 5A, B, D).
Moreover, further results showed miR-877 could be transferred from mechanical stretched-induced exosomes into recipient cells (BMSCs and HUVECs) and effectively expressed in recipient cells (BMSCs and HUVECs) (Fig. 5E–H).
Cyclic mechanical stretch-induced ADSCs-exosomes promote osteogenesis differentiation and angiogenesis to enhance bone healing via upregulating miR-877 expression in vitro.
To certify the effect of miR-877 on osteogenesis differentiation and angiogenesis in vitro, exogenous synthetic miRNA-877 was introduced through lentivirus vector transfection. First, the effect of miR-877 on BMSCs osteogenesis differentiation was investigated through BMSCs transfected with miR-877-mimic, mimic-NC, miR-877-inhibitor or inhibitor-NC, respectively. The mimic-NC group served as the control, and intergroup comparisons were performed. qRT-PCR results showed that miR-877-mimic increased the levels of miR-877 in BMSCs, while miR-877-inhibitor significantly decreased the levels of miR-877 in BMSCs (Fig. 5D). Next, the protein levels of RUNX2 and OCN expressions in the indicated BMSCs were determined by Western blotting. The results showed a significantly increased expression in these osteogenesis-related genes in the miR-877-mimic group, but a decreased expression occurred in miR-877-inhibitor group (Fig. 6G–J). Moreover, Alizarin red and ALP staining revealed that mineralization was significantly increased after miR-877 overexpression, while decreased following miR-877 inhibition (Fig. 6A).
Exogenous synthetic miR-877 transfected to BMSCs and HUVECs has the effects of significantly enhancing osteogenesis in BMSCs and angiogenesis in HUVECs respectively. A The effect of miR-877 on BMSCs osteogenesis differentiation transfected with miR-877-mimic, mimic-NC, miR-877-inhibitor or inhibitor-NC, respectively. B The effect of miR-877 on HUVECs angiogenesis after transfected with miR-877-mimic, mimic-NC, miR-877-inhibitor or inhibitor-NC respectively. C, D Confirmation of the transfection efficiency of miR-877 in BMSCs and HUVECs. E, F Quantitative and statistical analysis in each group of osteogenesis and angiogenesis. G–J The protein levels of osteogenesis gene (RUNX2 and OCN) expressions in the indicated BMSCs were determined by Western blotting. K–N Western Blot analysis was conducted to evaluate the effects of angiogenesis related proteins among all groups (Full-length blots/gels are presented in Supplementary Fig. 4). Data are presented as the mean ± SD, and all experiments were repeated three times (* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001; ns p > 0.05, not significant)
Furthermore, the effect of miR-877 on HUVECs angiogenesis was investigated through HUVECs transfected with miR-877-mimic, mimic-NC, miR-877-inhibitor or inhibitor-NC, respectively. First, qRT-PCR results showed that miR-877-mimic increased the levels of miR-877 in HUVECs, while miR-877-inhibitor significantly decreased the levels of miR-877 in HUVECs (Fig. 6C, D). After miR-877 was successfully transfected, the protein expression levels of VEGF and CD31 in the transfected HUVECs were determined by Western blotting assay. The results also showed a significantly increased expression in these angiogenesis-related genes in miR-877-mimic group, but a decreased expression occurred in miR-877-inhibitor group (Fig. 6K–N). Moreover, tube forming assay revealed the angiogenesis effect was significantly increased after miR-877 overexpression, while pronouncedly decreased following miR-877 inhibition (Fig. 6B).
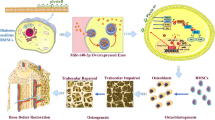
In short, cyclic mechanical stretch-induced ADSCs-exosomes promote osteogenesis differentiation and angiogenesis to enhance bone healing via upregulating miR-877 expression in vitro (Scheme 1).
Discussion
Exosomes as multiplexed signaling platforms are able to transmit abundant cargo molecules containing complex autocrine and paracrine signals. This pathway of intercellular vesicle traffic plays critical modulatory roles in many aspects of human health and disease, including development, immunity, tissue homeostasis, cancer, and neurodegenerative diseases. Based on these properties, exosomes are being developed as novel therapeutic agents in multiple disease models [29].
Mechanical stimulation is a crucial factor in bone development, regeneration and fracture healing. When subjected to mechanical stimulations, osteoblasts show numerous cellular responses that are of potential relevance to fracture healing, including proliferation, production of alkaline phosphatase, differentiation, secretion of extracellular matrix proteins, and mineralization, which presumably result from cellular reactions to the signaling molecules and changes in gene expression patterns. In this study, one-way elongation of different magnitudes, the effects of cyclic mechanical stretch induced ADSCs-exosomes on BMSC, including cellular proliferation, osteogenic differentiation, ALP activity, and mRNA level of osteogenesis-related genes under different magnitudes of cyclic mechanical strain were examined. The results revealed BMSC respond to exosomes derived from mechanical stress treated ADSC in a magnitude-dependent manner. Lower magnitude mechanical stretch stimulated ADSC exosomes (1.0 Hz, 6% mechanical stretch ADSCs, LMS-Exos) promote cellular proliferation, osteogenesis differentiation in the cellular and molecular levels, which were congruous with previous research [19, 30]. Further experiments proved that lower magnitude mechanical stretch (1.0 Hz, 6% mechanical stretch ADSCs, LMS-Exos) could improve bone regeneration and fracture healing in a rat non-union model. while higher magnitude mechanical stretch (1.0 Hz, 18% mechanical stretch ADSCs, LMS-Exos) showed inhibitory effects on cellular proliferation, osteogenesis differentiation in the cellular and molecular level, and ultimately impede fracture healing in a rat non-union model.
To elucidate the molecular mechanisms underlying the response of BMSCs to mechanical strains comparable to those occurring during bone regeneration and fracture healing, ADSCs were stretched to obtain mechanical stretch-induced exosomes in vitro. MicroRNAs (miRNAs) are 20–25 nucleotide (nt) non-coding RNAs that serve as post-transcriptional modulators of multiple physiological processes [31, 32]. Given that miRNAs are crucial bioactive molecules contained in exosomes [33, 34], then the exosomes secreted from different magnitude mechanical stretch-induced ADSC-MS-Exos were extracted, and followed by RNA sequencing, bioinformatics analysis, and qRT-PCR to evaluate miRNAs in ADSC-MS-Exos. The results suggested that miR-877 was the most significantly upregulated miRNA. Furthermore, the transfection of BMSCs and HUVECs with miR-877 mimics and inhibitors revealed that miR-877 promotes bone regeneration and fracture healing in vitro and in vivo. This is because miR-877 enhances BMSC proliferation, osteogenesis differentiation, and endothelial cell migration and angiogenesis.
However, fracture healing and bone regeneration are complex biological processes that involve many types of special cells and their secreted substances, bioactive molecules at different stages [35, 36]. Moreover, current studies have confirmed that different forms and magnitudes of mechanical stimuli may directly or indirectly regulate those processes, resulting in different or even paradoxical conclusions [37, 38]. Thus, mechanical factors transduction and regulatory pathways may be sophisticated interactive crosstalk which are not fully understood. Recently, there have been a few studies of miRNA-877, which mainly have found that miRNA-877 could affect invasion and metastasis of cancers or carcinoma, osteoblast differentiation and their possible underlying mechanism. For instance, Zhu et al. demonstrated that miR-877-5p alleviates chondrocyte dysfunction in osteoarthritis models by repressing the transcription factor FOXM1, which is known to regulate cell proliferation and inflammation pathways involved in cartilage degradation [39]. This finding suggests a protective role of miR-877-5p in cartilage homeostasis and osteoarthritic progression. Similarly, Shen et al. reported that miR-877-5p promotes osteoblast differentiation by directly targeting EIF4G2, a factor implicated in translational regulation, thereby facilitating osteogenic gene expression and bone formation [40]. These studies highlight the regulatory potential of miR-877-5p in musculoskeletal tissue remodeling, although its involvement in fracture healing, particularly under diabetic conditions, remains unexplored.
Our study presents a novel finding that transplantation of LMS-ADSC-Exos exerts an accelerated effect on bone regeneration and fracture healing by promoting osteogenesis and angiogenesis via miR-877 in diabetic rats. However, there are some limitations in our study. First, whether there exist any upstream signal pathways, such as circRNAs which usually act as ceRNAs and molecular sponge of miRNA involving mechanical stimulation induced exosomal miRNA-877 has not been investigated in our present study. Second, the definite and detailed downstream mechanisms of LMS-ADSC-Exos exert an accelerated effect on bone regeneration and fracture healing by promoting osteogenesis and angiogenesis via miR-877 have not been revealed, respectively. Third, while our findings are promising in a diabetic rat model, interspecies differences in bone biology may limit direct clinical extrapolation. Moreover, large-scale production, standardization, and quality control of mechanically stimulated ADSC exosomes pose practical hurdles for therapeutic application.
Therefore, the downstream or upstream signal pathways of mechanical stimulation-induced exosomal miRNA-877 still deserve further focus of research in the future.
Conclusion
In summary, the recent study has suggested exosomal miR-877 derived from mechanical stretch-induced ADSC could accelerate bone regeneration and fracture healing in T2DM diabetic rats via promoting osteogenesis differentiation and angiogenesis. The current finding shall provide new insights into the molecular mechanism of bone regeneration and fracture healing, and the development of new exosome-based remedy strategies for nonunion patients with diabetes.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Raw data can be found as supplementary material.
References
Wildemann B, Ignatius A, Leung F, Taitsman LA, Smith RM, Pesantez R, et al. Non-union bone fractures. Nat Rev Dis Primers. 2021;7(1):57.
Collaborators GBDF. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021;2(9):e580–92.
Zura R, Xiong Z, Einhorn T, Watson JT, Ostrum RF, Prayson MJ, et al. Epidemiology of fracture nonunion in 18 human bones. JAMA Surg. 2016;151(11):e162775.
Henderson S, Ibe I, Cahill S, Chung YH, Lee FY. Bone quality and fracture-healing in Type-1 and Type-2 diabetes mellitus. J Bone Joint Surg Am. 2019;101(15):1399–410.
Gortler H, Rusyn J, Godbout C, Chahal J, Schemitsch EH, Nauth A. Diabetes and healing outcomes in lower extremity fractures: a systematic review. Injury. 2018;49(2):177–83.
Alonso-Alonso ML, Garcia-Posadas L, Diebold Y. Extracellular vesicles from human adipose-derived mesenchymal stem cells: a review of common cargos. Stem Cell Rev Rep. 2022;18(3):854–901.
Nguyen TT, Corvera S. Adipose tissue as a linchpin of organismal ageing. Nat Metab. 2024;6(5):793–807.
Gavalda-Navarro A, Villarroya J, Cereijo R, Giralt M, Villarroya F. The endocrine role of brown adipose tissue: an update on actors and actions. Rev Endocr Metab Disord. 2022;23(1):31–41.
Cinti S. The endocrine adipose organ. Rev Endocr Metab Disord. 2022;23(1):1–4.
Li C, Wei S, Xu Q, Sun Y, Ning X, Wang Z. Application of ADSCs and their exosomes in scar prevention. Stem Cell Rev Rep. 2022;18(3):952–67.
An Y, Lin S, Tan X, Zhu S, Nie F, Zhen Y, et al. Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Prolif. 2021;54(3):e12993.
Bai Y, Han YD, Yan XL, Ren J, Zeng Q, Li XD, et al. Adipose mesenchymal stem cell-derived exosomes stimulated by hydrogen peroxide enhanced skin flap recovery in ischemia-reperfusion injury. Biochem Biophys Res Commun. 2018;500(2):310–7.
Ying C, Wang R, Wang Z, Tao J, Yin W, Zhang J, et al. BMSC-exosomes carry mutant HIF-1alpha for improving angiogenesis and osteogenesis in critical-sized calvarial defects. Front Bioeng Biotechnol. 2020;8:565561.
Zhao Q, Zhang Y, Xiao L, Lu H, Ma Y, Liu Q, et al. Surface engineering of titania nanotubes incorporated with double-layered extracellular vesicles to modulate inflammation and osteogenesis. Regen Biomater. 2021;8(3):rbab010.
Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–79.
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020. https://doi.org/10.1126/science.aau6977.
Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487–514.
Zhang J, Liu X, Li H, Chen C, Hu B, Niu X, et al. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res Ther. 2016;7(1):136.
Tschumperlin DJ, Liu F, Tager AM. Biomechanical regulation of mesenchymal cell function. Curr Opin Rheumatol. 2013;25(1):92–100.
Liu QY, Yu YF, Liu CJ, Liu YH, Yuan LJ, Wang Z, et al. Effect of La and Mg combined system on bioactivity and osteogenesis of bioinspired La- doped magnesium phosphate composites prepared utilizing the precursor method. J Mater Res Technol. 2023;24:9523–36.
Kowluru RA. Retinopathy in a diet-induced type 2 diabetic rat model and role of epigenetic modifications. Diabetes. 2020;69(4):689–98.
Du J, Chu Y, Hu Y, Liu J, Liu H, Wang H, et al. A multifunctional self-reinforced injectable hydrogel for enhancing repair of infected bone defects by simultaneously targeting macrophages, bacteria, and bone marrow stromal cells. Acta Biomater. 2024;189:232–53.
Sohrabipour S, Sharifi MR, Talebi A, Sharifi M, Soltani N. GABA dramatically improves glucose tolerance in streptozotocin-induced diabetic rats fed with high-fat diet. Eur J Pharmacol. 2018;826:75–84.
Furman BL. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol. 2015;70:5 47 41-45 47 20.
Wang JH, Thampatty BP. An introductory review of cell mechanobiology. Biomech Model Mechanobiol. 2006;5(1):1–16.
Yourek G, McCormick SM, Mao JJ, Reilly GC. Shear stress induces osteogenic differentiation of human mesenchymal stem cells. Regen Med. 2010;5(5):713–24.
Jagodzinski M, Drescher M, Zeichen J, Hankemeier S, Krettek C, Bosch U, et al. Effects of cyclic longitudinal mechanical strain and dexamethasone on osteogenic differentiation of human bone marrow stromal cells. Eur Cell Mater. 2004;7:35–41; discussion 41.
Liu C, Liu Y, Yu Y, Huang S, Sun C, Zhang D, et al. High glucose-induced senescent fibroblasts-derived exosomal miR-497 inhibits wound healing by regulating endothelial cellular autophagy via ATG13. Anal Cell Pathol (Amst). 2025;2025:8890200.
Connor DE, Paulus JA, Dabestani PJ, Thankam FK, Dilisio MF, Gross RM, et al. Therapeutic potential of exosomes in rotator cuff tendon healing. J Bone Miner Metab. 2019;37(5):759–67.
Shi Y, Fu Y, Tong W, Geng Y, Lui PP, Tang T, et al. Uniaxial mechanical tension promoted osteogenic differentiation of rat tendon-derived stem cells (rTDSCs) via the Wnt5a-RhoA pathway. J Cell Biochem. 2012;113(10):3133–42.
Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, et al. The 21-nucleotide RNA regulates developmental timing in. Nature. 2000;403(6772):901–6.
Bartel DP. Metazoan microRNAs. Cell. 2018;173(1):20–51.
Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19(1):92–105.
Zhang L, Ouyang P, He G, Wang X, Song D, Yang Y, et al. Exosomes from microRNA-126 overexpressing mesenchymal stem cells promote angiogenesis by targeting the PIK3R2-mediated PI3K/Akt signalling pathway. J Cell Mol Med. 2021;25(4):2148–62.
Hu Y, Tang L, Wang Z, Yan H, Yi X, Wang H, et al. Inducing in situ M2 macrophage polarization to promote the repair of bone defects via scaffold-mediated sustained delivery of luteolin. J Control Release. 2024;365:889–904.
Wang Z, Chu Y, Du J, Hu Y, Wang H, Liu H, et al. Accelerating repair of infected bone defects through post-reinforced injectable hydrogel mediated antibacterial/immunoregulatory microenvironment at bone-hydrogel interface. Carbohydr Polym. 2025;351:123082.
Bahney CS, Zondervan RL, Allison P, Theologis A, Ashley JW, Ahn J, et al. Cellular biology of fracture healing. J Orthop Res. 2019;37(1):35–50.
Einhorn TA, Gerstenfeld LC. Fracture healing: mechanisms and interventions. Nat Rev Rheumatol. 2015;11(1):45–54.
Zhu S, Deng Y, Gao H, Huang K, Nie Z. Mir-877-5p alleviates chondrocyte dysfunction in osteoarthritis models via repressing FOXM1. J Gene Med. 2020;22(11):e3246.
Shen Y, Zhang Y, Wang Q, Jiang B, Jiang X, Luo B. Microrna-877-5p promotes osteoblast differentiation by targeting EIF4G2 expression. J Orthop Surg Res. 2024;19(1):134.
Acknowledgements
The authors declare that they have not used AI-generated work in this manuscript.
Funding
This work was supported by the supported by the National Natural Science Foundation of China (No. 82072440), the Science and Technology Innovation Cultivation Fund of Zhongnan Hospital of Wuhan University (No. CXPY2023028), Excellent Doctor Fund Project of Zhongnan Hospital of Wuhan University (No. ZNYB2022015), Natural Science Foundation of Hubei Province (No.2022CFB699; No. 2024AFD167), the China Postdoctoral Science Foundation (No. 2023M742701).
Author information
Authors and Affiliations
Contributions
Aixi Yu, Chao Jian conceptualized and designed the overall experimental plan. Aixi Yu, Chao Jian, Liang Tian and Dong Zhang carried out all the experiments and data analysis with support from Zheng Wang, Junwei Liu and Changjiang Liu. Aixi Yu, Chao Jian, Liang Tian and Dong Zhang wrote the manuscript and prepared figures. Aixi Yu, Chao Jian, Liang Tian, Dong Zhang, Zheng Wang, Junwei Liu and Changjiang Liu analyzed and interpreted the data. Zheng Wang, Junwei Liu and Changjiang Liu provided reagents/materials/analysis tools. Aixi Yu, Chao Jian and Dong Zhang provide funding acquisition. Chao Jian and Dong Zhang critically reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Rats in this study were purchased from the laboratory Animal Center of Wuhan University (China). All animal experiments were performed under the guidance of the Institutional Animal Care and Use Committee (IACUC) of Wuhan University. The experimental protocol was approved by the Committee on the Ethics of Animal Experiments of Wuhan University, Wuhan, China (Title of the approved project: "Research on the Role and Mechanism of Stress-Induced Adipose-derived Stromal cells in Promoting Bone Healing." Approval number: ZN2024028; Date of approval: April.1st.2024.). All efforts were made to minimize animal suffering. The human cells (HUVECs) were separated from human umbilical vein vascular wall, the HUVECs (PUMC-HUVEC-T1; Cat NO: CL-0675) used in our experiments were originally purchased from a commercial vendor (Wuhan Pricella Biotechnology Co.Ltd. Wuhan, China). The original source has confirmed that there was initial ethical approval for collection of human cells, and that the donors had signed informed consent (https://www.procell.com.cn/).
Consent for publication
Not applicable.
Competing interests
The authors declared no potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tian, L., Zhang, D., Wang, Z. et al. MiR-877, an exosomal miRNA from mechanical stretch induced adipose derived stromal cells, enhances fracture healing in nonunion rats with type 2 diabetes mellitus. Stem Cell Res Ther 16, 575 (2025). https://doi.org/10.1186/s13287-025-04692-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-025-04692-w