Abstract
Background
Precision fermentation offers a sustainable alternative production route for proteins but still suffers from moderate productivities and low yields. Especially compared to biomass yields, recombinant protein yields on substrate are very low. Uncoupling recombinant protein production from growth would allow higher product yields, but requires that productivity is maintained. So far, two-phase production processes mostly rely on inducers to activate recombinant protein production after an initial growth phase, e.g., a change in carbon source. On large scale, specific growth rates can be controlled by nutrient availability, and we aim to use this as trigger to uncouple recombinant protein production from growth.
Results
We investigated the correlation between low specific growth rates (0.02 h− 1 < µ < 0.1 h− 1) and specific recombinant protein production rates, both for intracellularly accumulating and secreted proteins. By comparing two differently regulated promoters, the strong, constitutive PTEF1 and stress-induced PHSP12, we show that recombinant protein production rates and yields in Saccharomyces cerevisiae can be partially uncoupled from growth. The optimal strategy thereby differs for intracellular and secreted production. The PHSP12 resulted in increased product yields of intracellular protein at very low growth rates, including a 10-fold increase in intracellular protein titer, while titers remained virtually constant for the benchmark PTEF1. The PTEF1 on the other hand led to increased protein secretion rates and efficiencies at lower specific growth rates cumulating in higher extracellular protein titers.
Conclusion
Our results demonstrate that promoter selection plays a critical role in production performance under slow growing conditions. Moreover, it highlights that optimising intracellular and extracellular recombinant protein production requires distinct, strategy-specific approaches.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12934-025-02848-0.
Keywords: Yeast, General stress response, Recombinant protein production, Protein secretion limitation, Slow growth, Growth uncoupling
Background
Climate change and sustainable food security are urgent global challenges that demand innovative and efficient solutions to reduce our environmental impact, while safeguarding and improving food production. One promising solution to these challenges is precision fermentation, a biotechnological process that uses microorganisms to produce high-quality food ingredients. By precisely controlling microbial metabolism, this approach allows for the biosynthesis of specific compounds, such as dairy proteins, without relying on traditional livestock farming [1]. Despite significant advancements in microbial fermentation for industrial bioprocesses, challenges remain, especially for cost-effective recombinant protein production. One of the main bottlenecks is low product yield, i.e., the amount of protein produced per substrate consumed. To reach maximum yields, all substrate should be used for the product-of-interest. However, in precision fermentation processes, including those employing yeast cells, a large fraction of substrate is lost to biomass formation, or, in other words, to growth.
Different strategies can be employed to reduce growth. Most commonly, nutrient limitation is used to reduce the specific growth rate of yeast. Such strategies could result in high product yields, but so far these have only been achieved for catabolic products such as ethanol. For example, Boender et al. cultivated Saccharomyces cerevisiae at near-zero growth rates under anaerobic conditions and reached ethanol yields above 90% of the theoretical maximum [2]. Such high catabolic yields can be reached, because cells rely on product formation for cellular energy and, also under non-growing conditions, cells need energy to remain alive, the so-called maintenance energy requirement [3]. This is however different for anabolic products, such as recombinant proteins. Their formation is often directly linked to growth and requires a net input of energy. Hence, merely limiting growth does not automatically increase product yields: This also requires that productivities are maintained or, at least, not as strongly reduced as growth rate is, hence strategies to uncouple product formation from growth are needed.
To investigate the correlation between specific growth rate (µ) and product formation, typically chemostat cultures are employed. These continuous cultures give control over the specific growth rate and have - for example - been used to find a strong growth-rate dependency for resveratrol production in S. cerevisiae [4]. Retentostat cultivation, also known as perfusion cultivation, allows a controlled transition from slow to near-zero growth rates and can be used to study virtually non-growing microbial cultures, as well as understanding growth dynamics [5]. Retentostat cultures have also been used to study production dynamics at extremely low growth rates in yeast species. Komagataella phaffii, for example, sustained recombinant protein production in slow-growing retentostat cultures [6]. These approaches are promising to provide leads to uncouple growth from product formation but also show a strong growth-rate dependency of product formation rates. This means that, generally, specific production rates (qP) are extremely low in the absence of growth. Compared to chemostat and retentostat cultures, fed-batch cultures offer a more time-efficient method to study yeast physiology at decreasing growth rates, including correlations between specific growth and production rates. In a recent study we employed this approach to show differences in maintenance energy requirements between strains [7].
Previous studies have explored how yeast cells respond at the transcriptional level to low growth rates, uncovering distinct patterns of gene expression regulation [8–11]. For example, both protein and transcript levels of heat shock protein 12 (Hsp12) were found to show an inverse correlation with specific growth rate, i.e., levels increased when growth slowed down [5, 8, 12, 13]. Expression of HSP12 is in part controlled by the transcription factors Msn2/4 as part of the general stress response, which is also activated under carbon-limitation [11]. The promoter region of HSP12 was recently employed to express lipase B in K. phaffii, successfully inducing recombinant protein expression under carbon-limiting conditions [14]. Since HSP12 has similar induction patterns in S. cerevisiae, its promoter (PHSP12) is a strong candidate for decoupling recombinant protein production from growth.
The promoter of TEF1 (PTEF1), which regulates the expression of Translation Elongation Factor 1-alpha, is commonly considered a constitutive promoter for recombinant protein production in S. cerevisiae because of its stable expression level under various conditions [15–17]. However, the activity of the PTEF1 under slow-growing conditions remains unclear, especially whether it shows activity decoupled from the specific growth rate. By using PTEF1 to drive the expression of heterologous proteins, we investigate whether expression indeed remains stable in slow-growing cells or is reduced as the growth-rate decreases. This is essential for understanding the limitations of constitutive promoters in growth-decoupled systems and for evaluating whether stress-induced promoters, like PHSP12, provide a more reliable alternative for sustained protein expression during slow growth.
Protein secretion offers advantages in precision fermentation processes. Secretion simplifies downstream processing, thereby enhancing overall efficiency and scalability in industrial applications [18, 19]. Furthermore, it would also enable more effective decoupling of growth from protein production. This is because, cells could be kept in a secreting, but non-dividing state, as production is independent of cellular space and hence does not require increasing biomass levels in contrast to intracellular protein accumulation. Therefore, we examined whether correlations between specific intracellular protein production rates (qP) and specific growth rate (µ) also hold for specific protein secretion rates (qPex), aiming to understand how growth rate affects the amount of protein secreted.
Methods
Strains and strain construction
Strains ScNTi001, ScNTp001 and ScNTp002 were used in this study (Table 1). All strains were grown in appropriate media until late exponential phase and stored as glycerol stocks (30% v/v) at -80 °C. ScNTi001 was derived from CEN.PK113-7D [20], by integration of the dual expression cassette (PHSP12-mRuby2-ttADH1 - PTEF1-ymNeongreen-ttTDH1) in the X-2 locus [21]. ScNTp001 and ScNTp002 are derived from CEN.PK113-5D [20] by transforming a multicopy (2µ) plasmid carrying the klURA3 gene and a ymNeongreen secretion cassette driven by the TEF1 or HSP12 promoter. All plasmids and primers used are shown in Supplementary Tables 1 and 2, respectively.
Table 1.
S. cerevisiae strains used in this study
| Species | Strain | Genotype | Reference |
|---|---|---|---|
| S. cerevisiae | CEN.PK113-7D | MATa, MAL2-8c, SUC2 | [20] |
| S. cerevisiae | CEN.PK113-5D | MATa; ura3-52; MAL2-8C; SUC2 | [29] |
| S. cerevisiae | ScNti001 | CEN.PK113-7D X-2::PHSP12-mRuby2-TADH1:PTEF1- ymNeonGreen-TTDH1 | This study |
| S. cerevisiae | ScNtp001 | CEN.PK113-5D + pPTEF1-SPKsh1-ymNeongreen | This study |
| S. cerevisiae | ScNtp002 | CEN.PK113-5D + pPHSP12-SPKsh1-ymNeongreen | This study |
To incorporate the promoter region, the 1 kb upstream sequence of the HSP12 start codon was synthesized (IDT, USA), with overhangs for integration into the Yeast Tool Kit [22]. The HSP12 promoter fragment was cloned into the entry plasmid (YTK001) via Golden Gate cloning, followed by E. coli transformation and purification. The yeast-optimized mNeongreen fluorescent protein coding sequence with specific overhangs was amplified from a ymNeongreen plasmid (addgene #125704) from [23] using PCR with primers designed to incorporate the desired overhang sequences. The PCR product was assembled into the entry plasmid (pYTK001) using Golden Gate cloning to generate a pYTK-ymNeongreen part plasmid. For protein secretion, we fused the Ksh1 secretion signal sequence [24] and ymNeongreen coding sequence. The Ksh1 secretion signal peptide (SPKsh1) and ymNeongreen coding sequences were amplified separately, while ensuring seamless fusion between them and compatibility with the entry part plasmid (YTK001). The SPKsh1 coding sequence was ordered as two long complementary primers (IDT, USA), while ymNeongreen was amplified from the pYTK-ymNeongreen plasmid. The annealed oligonucleotide duplex and PCR product were assembled into the entry plasmid (YTK001) resulting in pYTK-SPKsh1-ymNeongreen.
The intracellular fluorescence protein-expressing strain ScNti001 was constructed from the prototrophic strain S. cerevisiae CEN.PK113-7D. Using Golden Gate cloning, a reporter plasmid was assembled containing two transcription units: PHSP12-mRuby2-TADH1, and PTEF1-ymNeonGreen-TTDH1. This plasmid was used as to PCR amplify the integration cassette consisting of both transcription units. Integration into the X-2 region was facilitated by a single CRISPR-Cas9 plasmid encoding the spCas9 gene and a gRNA targeting the locus on chromosome X. This plasmid was constructed following the approach of Juergens et al., based on plasmid pUDP002 (Addgene #103872) [25, 26]. Successful integration was verified via colony PCR. The CRISPR-Cas9 plasmid was cured by culturing the strains in YPD without selective pressure.
For protein-secretion, S. cerevisiae strain CEN.PK113-5D was transformed with plasmid pPHSP12-SPKsh1-ymNeongreen or pPTEF1-SPKsh1-ymNeongreen. Multicopy plasmids with the URA3 selection marker were constructed from part plasmids using Golden Gate cloning [22]. Plasmids were validated by restriction analyses and sequencing.
Yeast transformations were carried out using the lithium acetate method [27]. YPD media with 200 µg/mL hygromycin were used as selective pressure for integration, synthetic medium [28] lacking uracil was used for selection of cells carrying the plasmid.
Fed-batch cultivations
Shake-flask precultures for fed-batch experiments were grown in 500 mL shake flasks containing 100 mL of 6.7 g/L YNB medium (Y0626 Sigma Aldrich), set to pH 6.0 with 2 M KOH before autoclaving and supplemented with 20 g/L glucose. These shake-flask cultures were inoculated with 2 mL of frozen stock culture and incubated in an orbital shaker at 200 rpm and 30 °C.
Fed-batch cultivations were performed in 3-liter bioreactors (Getinge/Applikon, Delft, the Netherlands). The operational parameters were set as follows: the temperature was maintained at 30 °C, the constant stirring speed was 1000 rpm, and sterile air was sparged at 900 mL/min. pH was controlled at 5.0 for intracellular protein experiments and 6.5 for secretion experiments by automatic addition of 2 M potassium hydroxide. The cultures for the fed-batch were grown in 6.7 g/L YNB with 3 g/L ammonium sulphate, 1.94 g/L yeast synthetic dropout supplement without uracil (Y1501 Sigma Aldrich), 0.02 g/L BSA, 1 mL/L antifoam B (Sigma-Aldrich) and 17 g/L glucose for the batch phase. The feed medium consisted of 6.7 g/L YNB with 3 g/L ammonium sulfate, 3.88 g/L yeast synthetic dropout supplement without uracil, 0.02 g/L BSA, 1 mL/L antifoam B (Sigma-Aldrich) and 70 g/L glucose. Feeding started once extracellular carbon sources were exhausted during the batch phase, with a constant feed rate of 30 mL/h.
Determination of substrate and biomass concentrations and off gas composition
The culture dry weight was determined by filtering precisely 10 mL of an appropriately diluted culture broth through pre-dried and pre-weighed membrane filters (Gelman Science). Samples were diluted with demineralised water when biomass concentration was higher than 15 g/L, prior to culture dry weight assays. The filters were then washed with demineralized water, dried for 20 min at 350 W in a microwave oven, and reweighed [30]. Supernatants for sugar analysis were prepared by centrifuging culture samples for 5 min at 16,000×g. These supernatants and media were analysed in a YSI Glucose Analyzer (2950 Biochemistry Analyzer, Yellow Spring Instruments). OD was measured at 600 nm in determined time intervals using a Hach Lange DR600 spectrophotometer. The exhaust gas from the fed batch cultures was cooled using a condenser set at 4˚C and subsequently dried. The dried gas was then analysed for carbon dioxide, oxygen and ethanol concentrations using a mass spectrometer (Prima δB, Thermo Fisher).
Determination of intracellular relative fluorescent protein levels and viability
Aliquots of 1 mL culture broth were diluted to 1 × 107 with PBS buffer pH 7.5 (VWR). Fluorescence intensity was measured using a Cytoflex flow cytometer (Beckman Coulter, the Netherlands), excited with a 488 nm laser. ymNeongreen fluorescence was measured with a 525/40 nm filter, while mRuby2 fluorescence was measured with a 610/20 nm filter. In addition, culture viability was assayed by staining 1 × 107 cells with 4 µg/mL propidium iodide (PI) as described previously [31]. After 15 min incubation in the dark, fluorescence of the samples was measured with a Cytoflex flow cytometer (Beckman Coulter, the Netherlands), excited with 488 nm, and measured with a 690/50 nm filter. A total of 10,000 cells were counted for each sample. Prior to sample acquisition, the cytometer was calibrated using fluorescent calibration beads to ensure consistent detector performance across experiments. A threshold was based on forward scatter (FSC-A) to exclude small particles and background noise. Cells were gated based on FSC-A versus side scatter (SSC-A) to exclude debris. PI-positive and PI-negative populations were distinguished based on 690/50 nm fluorescence intensity versus FSC-A plots. Flow cytometry data were analysed using FlowJo v10.8.1 software (BD Biosciences, Franklin Lakes, NJ, USA).
Determination of secreted fluorescence protein intensity
1 mL aliquots of culture broth were centrifuged at 5000 g for 5 min. 200 µL of supernatant was placed into a black 96 well plate (Nunc, Thermo Fisher) and analysed for green fluorescence. Measurements were performed in a Synergy H1 plate reader (Biotek) with monochromators set to the excitation wavelength of 488 nm and emission at 507 nm.
Calculation of fed-batch growth kinetics
Titers of intracellular and extracellular fluorescent protein were calculated using Eqs. (1) and (2), respectively. In Eq. (1), TIP denotes intracellular protein titer (a.u./L), fluorescence intensity is the mean fluorescent intensity detected using flow cytometry (a.u.) corrected for mean cell size (based on FSC-A, a.u.), cX is the biomass concentration (g/L). In Eq. (2), TEP denotes extracellular protein titer (a.u./L), Fluorescence intensity is the measured fluorescence intensity of the supernatant (a.u./L).
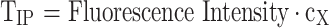 |
1 |
 |
2 |
Specific growth rates (µ in h− 1) were calculated as previously described [7]. Specific protein production rates (qP in a.u./g/h) were calculated using Eq. (3). In this equation rP denotes the protein production rate at the time t (h), MX is the biomass amount (g) in the reactor at the time t. Biomass amounts were calculated based on Eq. (4). Protein production rates were calculated by determining the slope of protein amount (i.e., Titer x Volume) versus time. For first-order kinetics, the rate corresponds to the linear slope of protein amount versus cultivation time. For second-order behaviour, the rate was derived from the time-dependent change in the slope, calculated as the first derivative of the fitted curve, where time was the independent variable.
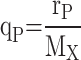 |
3 |
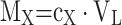 |
4 |
Protein yield on substrate (YPS) at the time point t was calculated with Eq. (5), where qS is the specific substrate consumption rate (g/g/h) estimated with a previously described model [7].
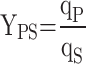 |
5 |
All normalizations were performed against the value at the start of the feeding phase. For strains ScNTp001 and ScNTp002 the average value of both strains at the start of the feeding phase was used for normalisation. As a result, all normalized values represent relative amounts and are unit-less.
Statistical analyses
All statistical analyses were performed using GraphPad Prism (version 10.4.1, Dotmatics). Cell size comparisons between strains were evaluated using unpaired t-tests and simple linear regression. Correlation of specific protein production rates (qP) or secretion rates (qpex) with growth rate (µ) were analyzed using Pearson correlation to assess linear relationships, with significance set at p-value < 0.05. The relationship between secretion rates (qpex) and intracellular fluorescence intensity was evaluated using Spearman correlation. Biomass yield on substrate (YXS) comparisons between strains were analyzed using a paired t-test. All statistical tests were conducted with a significance threshold of p-value < 0.05.
Results
Effect of intracellular protein expression on physiology
Fed-batch cultivation is a semi-continuous method in which feed is continuously added without removing any culture. This approach enables high cell densities and product titers in a controlled environment, making it popular in industrial settings [32, 33]. Different feeding strategies can lead to varying growth characteristics in fed-batch cultures. We maintained a constant feed flow rate to gradually decrease the specific growth rate over time. This allowed us to study how productivity varies with growth rate when employing stress-responsive (PHSP12) or constitutive (PTEF1) promoters.
During the feeding phase, the initial specific growth rate (µ) was 0.081 ± 0.003 h⁻¹, which gradually decreased to 0.015 ± 0.001 h⁻¹ over 42 h (Fig. 1A). At the end of the cultivation, the biomass yield reached 0.32 ± 0.01 g/g. Using Pirt’s model we estimated the distribution of consumed glucose (qS) between growth (µ), protein formation (qp), and non-growth associated processes (mS). The non-growth associated glucose consumption was estimated at 0.017 ± 0.001 g/g/h, corresponding to 37.0 ± 0.0% of the glucose consumed at the end of the fed-batch culture. As the product accumulated intracellularly this value includes both the cellular maintenance requirement and part of the glucose allocated to product formation under non-growing conditions. Cell viability remained above 98% (Supplementary Fig. 1A), while the average cell size increased significantly during the cultivation, leading to app. 1.5-fold larger cells (t-test p-value = 0.0041, Supplementary Fig. 1B).
Fig. 1.
Protein expression and growth characteristics of ScNti001 during fed-batch cultivation. a Cellular fluorescence intensity (a.u./cell) (left axis) - normalized to the average value (a.u./cell) at the start of feeding phase - and growth rate (h− 1) (continuous line, right) over time; b Specific protein production rate (qp) (a.u./g/h) - normalized to the average value (a.u./g/h) at the start of feeding phase - throughout the cultivation, dashed lines correspond to linear regression results; c Intracellular fluorescent protein titer (a.u./L) as function of fed-batch culture age; d Protein yield on glucose (a.u./g) at different growth rates – normalized to the average value (in a.u./g) at the start of feeding phase -. Green open triangles represent PTEF1-driven ymNeongreen; red closed circles indicate PHSP12 -driven mRuby2. See materials and methods for more details on normalization and units
Expression driven by TEF1 promoter shows a positive correlation with the specific growth rate
Cellular fluorescence intensity caused by fluorescent protein expression was measured using flow cytometry (Fig. 2 and Supplementary Fig. 2). The cellular mRuby2 fluorescence intensity driven by the HSP12 promoter (PHSP12) and ymNeongreen fluorescence intensity controlled by the TEF1 promoter (PTEF1), showed opposite trends over time (Figs. 1A and 2). At the start of the feeding phase, all cells show medium to high green fluorescence (mean intensity = 60.4 ± 0.2 kAU), gradually decreasing to lower intensities (mean intensity = 17.0 ± 0.4 kAU). This implies that PTEF1 activity is correlated with the specific growth rate. Indeed, specific product formation rates (qP) show a positive, linear correlation with specific growth rate (µ) (Fig. 1B) (p < 0.0001, Pearson r = 0.9998). Extrapolation to a growth-rate of zero, predicts a significant growth-rate independent specific production rate (mP, p < 0.0001).
Fig. 2.
Histograms of intracellular fluorescence intensities per cell for strain ScNti001 during fed-batch cultivation. a ymNeongreen and (b) mRuby2 fluorescence intensities (a.u.) per cell. Y-axes indicate numbers of cells (per 10000) per time point. Corresponding growth rates are indicated as well. Data are shown for one culture, data for the replicate are shown in supplementary Fig. 2. Background control refers to data from S. cerevisiae strain CEN.PK 113- 7D that does not express any fluorescent proteins
While ymNeongreen protein levels per cell decreased, titers remained almost constant during the fed-batch cultures due to the increase in biomass concentration (Fig. 1C, Supplementary Fig. 1). In addition to biomass concentration, the culture volume increased, and the total amount of ymNeongreen protein increased over time. This indicates that ymNeongreen production continued, even though glucose availability was limited. This is also illustrated by a growth-rate-independent protein yield (Fig. 1D).
Expression driven by PHSP12 enables strong increase in intracellular protein titers
While the PTEF1 mediated protein production resulted in average cellular fluorescence intensities that gradually decreased, PHSP12 mediated protein production resulted in increased cellular protein levels with time and decreasing growth rate (Figs. 1A and 2). At the start of the feeding phase, only 12.6 ± 3.6% of the cells were highly fluorescent (i.e., with fluorescence intensities above 10kAU). After already 4 h of feeding (µ = 0.058 h− 1), fluorescence intensity increased dramatically with 81.7 ± 2.3% of cells exhibiting high fluorescence. By the end of the cultivation, this proportion increased further to 96.6 ± 1.0% (Fig. 2B and Supplementary Fig. 2). Combined with increasing biomass concentrations, these increased cellular levels represent an increase in mRuby2 titer (Fig. 1C). E.g., after 42 h of constant feeding, the mRuby2 titer had increased 10-fold.
Also, for mRuby2 specific production rates (qP) were calculated based on the change of intracellular protein levels. These still showed a positive correlation within the range of growth rates tested here (Fig. 1B). Yet, the correlation parameters between production and glucose consumption differed, which resulted in an increase in product yield at lower growth rates (Fig. 1D). Linear regression furthermore revealed a growth-rate independent specific production rate (mP) that was 2.67 times higher than for the PTEF1.
Impact of promoter on correlation between protein secretion and specific growth rate
Based on the strong increase in intracellular protein titers at decreasing specific growth rates when using PHSP12, we explored if this also translated to higher secreted protein titers. Protein secretion provides several advantages for industrial-scale production, including more straightforward downstream processing and reduced costs. The same fed-batch strategy was applied for strains equipped with a multicopy plasmid harbouring the ymNeongreen coding sequence, including the Ksh1 signal peptide sequence [24] with either PTEF1 (ScNTp001) or PHSP12 (ScNTp002). As reporter protein, only ymNeongreen was used to avoid interfering effects from differences in protein trafficking that could occur when comparing it with mRuby2. Ksh1 is a high-level surface display protein [34] and its secretion signal has been used before to secrete fluorescent proteins in S. cerevisiae efficiently [24]. For secreted proteins, the pH of the medium impacts the fluorescence stability. Notably, within 2 h, ymNeongreen loses over 50% of its fluorescence at pH 5.0, whereas at pH 6.5 it retains 90% of its fluorescence [23]. Therefore, to increase the stability of extracellular ymNeongreen fluorescence, the pH was maintained at 6.5 instead of 6, to be well above the in vivo pKa of 5.42 [23]. Furthermore, extracellular proteases can contribute to the degradation of secreted recombinant proteins, potentially reducing overall yield. To mitigate this, we supplemented the media with bovine serum albumin (BSA), a known stabilising agent that has been demonstrated to prevent proteolytic degradation in previous studies [35, 36]. During the feeding phase, the specific growth rate decreased from 0.14 ± 0.004 h⁻¹ to 0.019 ± 0.001 h⁻¹ for strain ScNTp001 (PTEF1), while for strain ScNTp002 (PHSP12) this was comparable from 0.11 ± 0.005 h⁻¹ to 0.018 ± 0.001 h⁻¹ (Fig. 3A).
Fig. 3.
Impact of promoter type on protein (ymNeongreen) secretion efficiency under slow-growing conditions. a Extracellular protein titer (left axis) (a.u./L) – normalized to the average value (a.u./L) for both strains at the start of the feeding phase - and growth rate (right axis, continuous lines) during fed-batch cultivation; b specific secretion rate at different growth rates (a.u./g/h) normalized to the average value at the start of feeding phase; c intracellular protein titers (a.u./L) - normalized to the average value (a.u./L) for both strains at the start of the feeding phase; d intracellular specific protein production rate (a.u./g/h) normalized to the average value at the start of feeding phase. Dark green triangles represent ScNTp001 (PTEF1); light green circles indicate ScNTp002 (PHSP12). Data for two replicates (open and closed symbols) are shown. See materials and methods for more details on normalization and units
During the first 25 h, the extracellular titer of ymNeongreen increased 2.6-fold for PHSP12 controlled protein expression. However, after 25 h, titers did not increase further, but stabilised (Fig. 3A). This contrasted with the intracellular production observed in the ScNti001 strain, in which accumulation continued for the PHSP12-driven protein (Fig. 1C). On the other hand, the PTEF1 initially resulted in lower extracellular titers, but extracellular ymNeongreen titers did not level off in the 2-day timespan, and final yields were 1.5-fold higher compared to the PHSP12 results (Fig. 3A, Supplementary Table 3).
When we checked the specific secretion rates (qpex), the qpex of strain ScNTp002 (PHSP12) showed a positive linear correlation with growth rate (Fig. 3B, p-value < 0.0001, Pearson r = 0.9996). Although a positive linear correlation was also observed for intracellular production of PHSP12 driven mRuby2 (Fig. 1B), the correlation for secretion differed notably: cells appeared to stop secreting the fluorescent protein at growth rates below 0.02 h− 1 (Fig. 3B). This is in line with the plateauing titers (Fig. 3A). Surprisingly, for ScNTp001 (PTEF1), the correlation between qpex and µ was inverted, suggesting that the specific product secretion rate increases with a decreasing growth rate (Fig. 3B). This correlation clearly differed from the one observed for intracellular production in the integrated strain (Fig. 1B).
To understand the differences between protein titers and qP -µ correlations between the intracellular accumulating strain (Fig. 1) and secreting strains (Fig. 3), we also monitored intracellular protein levels in the secreting strains. The intracellular ymNeongreen levels of ScNTp002 (PHSP12) were higher than of ScNTp001 (PTEF1) throughout the entire cultivation (Fig. 3C). However, intracellular ymNeongreen levels slightly decreased in ScNTp002 (PHSP12) after 25 h, which was not observed for ScNTp001 (PTEF1). Specific production rates (qp) based on these intracellular levels showed a positive correlation with the growth rate in both strains (Fig. 3D, p-value = 0.019, Pearson r = 0.8331 for ScNTp001 (PTEF1), p-value < 0.0001, Pearson r = 0.9856 for ScNTp002 (PHSP12), in line with observations for the intracellularly accumulating strain (Fig. 1B).
These results suggest that a low, but constant expression results in higher extracellular titers at lower growth rates, in contrast to high expression. This implies a bottleneck in the secretory pathway. Comparison of specific secretion rates (qpex) versus specific intracellular production rates showed clear differences in the secretion efficiencies between both strains (Fig. 4A). While for ScNTp002 (PHSP12) the secretion efficiency (ratio qPex/qP) decreased on average by 40%, for the PTEF1 it increased over 3-fold. Intracellular protein levels can also indicate a secretory bottleneck, as efficient secretion should result in lower intracellular accumulation. A positive trend between intracellular levels and specific secretion rates was observed when the TEF1-promoter controlled expression (p-value = 0.018, Spearman r = 0.71), indicating that higher intracellular accumulation drove secretion. The PHSP12 led to an opposite correlation, where high intracellular levels corresponded to lower specific secretion rates (p-value = 0.02, Spearman r = -0.70) (Fig. 4B).
Fig. 4.
a Specific secretion rates (qP ex ) versus specific intracellular protein production rates (qP); b. Specific secretion rates (qPex) as function of average intracellular protein levels (intensity). Triangles in dark green represent ScNTp001 (P TEF1); the circles in light green indicate ScNTp002 (PHSP12). Data for two replicates (open and closed symbols) are shown
Effect of protein expression and secretion on physiology
Final biomass concentrations, and especially biomass yields on glucose (YXS) were higher for ScNTp001 (PTEF1) (0.42 ± 0.03 g/g) compared to for ScNTp002 (PHSP12) (0.34 ± 0.050 g/g) (Supplementary Table 3, Supplementary Fig. 1C. This is in line with a higher non-growth associated glucose consumption by ScNTp002 (PHSP12) (0.018 ± 0.007 g/g/h) compared to ScNTp001 (PTEF1) (0.008 ± 0.003 g/g/h). This means that expression by PHSP12 leads to the allocation of 36.2 ± 10% of the available glucose to these processes, which include cellular maintenance and product formation, at the end of the fed-batch culture, while for PTEF1 mediated protein production this is only 17.6 ± 5.1%. To further assess the impact of the multicopy plasmid and protein secretion, we monitored viability. Viability remained above 99% for ScNTp001 (PTEF1), while PHSP12 driven expression resulted in a decline in viability, especially after 25 h of cultivation to 89.0 ± 3.1% (Supplementary Fig. 1A).
For both strains, two subpopulations with regards to cellular fluorescence intensity were observed at all time points (Supplementary Fig. 3). This separation into two populations is likely caused by the presence or absence of the plasmid, where cells containing the plasmid express ymNeongreen resulting in higher fluorescence intensities. However, 70% of the cells showed above background, green fluorescence at all the time points, indicating fairly good plasmid stability under the given conditions. PHSP12-based expression resulted in higher average intensities (Fig. 3C and Supplementary Figs. 3B, 5D), but also more variation in fluorescent intensity among cells was observed, than for PTEF1-based expression (Supplementary Fig. 3A, C).
Discussion
The promotor region of the Translation Elongation Factor 1 alpha (TEF1) encoding gene has been characterized and used for recombinant protein production in different fungi, including Trichoderma reesei, K. phaffii and S. cerevisiae [37–39]. It is especially used because it shows activity under various conditions, i.e., on different carbon sources and during different growth phases in batch cultures [40]. Nevertheless, its activity has been shown to be partially growth-rate dependent in both S. cerevisiae and K. phaffii [41–43]. Here, we confirmed that the specific rate of intracellular recombinant protein production (qP) driven by PTEF1 indeed shows a positive correlation with the specific growth rate (µ) (Figs. 1B and 3D). Specific intracellular production rates of ScNTp001 were up to two orders of magnitude larger than for PTEF1-driven expression in ScNTi001 (Supplementary Fig. 4), which is likely caused by the use of a multi-copy plasmid in ScNTp001 versus a single integration of the expression cassette in ScNTi001. Linear regression revealed significant growth-independent specific production rates (mP) for both strains, indicating that Tef1p plays a role in cellular maintenance and is actively expressed under those conditions. The constant ymNeongreen yield on glucose (Fig. 1D) suggests that PTEF1 is actually specific substrate-consumption rate (qS) dependent. This putative dependency may offer opportunities to uncouple recombinant protein production from growth, when growth is arrested, but carbon-source consumption is maintained.
Secretion of recombinant proteins offers advantages with regards to down-stream purification and especially for growth-uncoupled production. For these advantages we examined ymNeongreen secretion using the Ksh1 signal peptide [24] and the PTEF1. The specific rates of ymNeongreen secretion (qPex) show an inverse correlation with specific growth rate (Fig. 3B), which thereby differs from the correlation of intracellular production rates (qP, e.g., Fig. 3D). This indicates that the cellular secretion capacity did not become limiting at low growth rates when expression was driven by PTEF1, but rather that the secretion efficiency, i.e., the ratio of secreted vs. intracellular accumulated protein (qPex/qP) increased more than threefold. This inverse correlation between specific protein secretion and growth rate is in line with PTEF1 driven secretion of human serum albumin in K. phaffii observed by Stadlmayer and colleagues at higher specific growth rates [43].
PHSP12 is an interesting candidate to uncouple recombinant protein production from growth, as Hsp12 expression shows a negative correlation with specific growth rate [8]. However, to our knowledge only one study examined its potential in this context, in K. phaffii [14]. Here we showed that in S. cerevisiae, PHSP12 indeed results in increased intracellular ymNeongreen levels at low growth rates (Fig. 1). However, despite these increased intracellular levels, the correlation between specific intracellular production rates (qP) and specific growth rates was still positive (Fig. 1B). The correlation however differed from that observed for PTEF1 -driven expression in terms of yield on biomass (YPX) and especially for the growth-rate independent production (mP). This extrapolated intracellular production in the absence of growth is a significant 3.5-fold higher for PHSP12 compared to PTEF1 (0.23 ± 0.06 vs. 0.084 ± 0.015 au/g/h, t.test p-value = 0.0425). This different correlation between qP and µ also translates into increased product yields at low growth rates (Fig. 1D). Such increased product over substrate yields (YPS) are important from a bioprocess perspective as substrate can contribute significantly to production costs of low-value food proteins. Previously, different correlations between YPS and µ have been observed for energy-requiring products in yeasts, where YPS either decreased or remained constant (e.g., [4, 19]. Our results thus show that, using the PHSP12, further uncoupling of growth from production can be achieved. Thereby our results are in line with observations in K. phaffii using variants of the PHSP12 [14]. However, we also show that the PHSP12 leads to high intracellular levels of heterologous proteins at low growth rates, a feature that can aid in the uncoupling of product formation beyond recombinant proteins from growth. For example, the PHSP12 can be used to drive the expression of enzymes in production pathways that should only be activated at near-zero growth rates to avoid toxicity or other cellular stress.
The still positive correlation between qP and µ implies that the reduction in overall biosynthetic processes still limits specific production rates at near-zero growth. This correlation has been attributed to the correlation between the specific growth rate and intracellular concentrations of key metabolic intermediates such as pyruvate [44, 45]. However, low specific production rates may be compensated for by increasing biomass concentrations to reach sufficiently high volumetric production rates.
At low growth rates, the PHSP12 led to higher specific secretion rates (qpex) than the PTEF1 and also extracellular protein titers were initially higher in ScNTp002 (PHSP12) fed-batch cultures (Fig. 3A and C). However, at very low growth rates (< 0.03 h− 1) in prolonged fed-batch cultures, the stress-responsive promoter did not outperform the constitutive PTEF1 in terms of specific secretion rates nor extracellular protein titers in our set-up. In contrast to the inverse correlation observed between growth rate and specific secretion rates for the PTEF1, for PHSP12 this correlation was positive. Extrapolation furthermore suggested that below a growth rate of 0.0031 h− 1, protein secretion driven by PHSP12 would not occur. These low secretion rates at extremely low growth rates and the subsequent lower titers, could not be explained by translation rates per se: Specific intracellular production rates for the PHSP12 were high in the range of growth rates tested here (Fig. 3D). These observations, together with the reduced secretion efficiency and high intracellular ymNeongreen levels (Fig. 4), strongly suggest a bottleneck in the secretion when PHSP12 is used. Also, the slight decrease in intracellular ymNeongreen levels in ScNTp002 (PHSP12) (Fig. 3C) implies that translation rates are lower than the combined rates of secretion and dilution by cell growth, as intracellular protein amounts at the culture level still increased. This reduced translation could be caused by for example reduced transcription or cellular stress responses.
The secretion bottleneck could be caused by the combination of the multi-copy plasmid used for the expression cassette and the regulation of PHSP12. The multiple copies of the PHSP12-SPKsh1-ymNeongreen expression cassette result in high expression at low growth rates and hence a strong accumulation of recombinant protein. This is supported by the inverse correlation that we observed between intracellular protein levels and specific secretion rates and suggests a bottleneck in the secretory pathway (Fig. 4B). The intracellular levels reached are up-to 6 times higher compared to PTEF1 driven expression (Fig. 4B). The balance between translation and secretion rates is intricate and, when secretion becomes a limiting factor, cells experience stress. Maintaining the balance between translation and secretion at an optimal rate is therefore key to maximize overall protein production [46]. High intracellular concentrations of recombinant protein strain the protein folding and secretory machinery of S. cerevisiae, which can trigger the unfolded protein response (UPR) [47]. In yeast, the UPR is activated through Ire1p-mediated splicing of the HAC1 mRNA, which results in a functional Hac1 transcription factor [48]. Hac1 subsequentially activates and represses the expression of several proteins, both to increase the protein processing capacity and reduce translation rates [49]. Among the Hac1 regulon are chaperones and the PHSP12 also contains a Hac1 transcription factor binding motif (5′-RMCACGT-3′) (SGD, Yeastract). Hence the use of PHSP12 for recombinant protein secretion could result in a negative feedback loop: High expression driven by PHSP12 activates the UPR, which subsequently further activates PHSP12. This negative feedback loop would result in increased cellular stress and reduced performance. Misfolded proteins and aggregates have previously been shown to activate cellular stress responses, which can ultimately limit the ability to sustain specific protein secretion [48].
Physiological differences observed between the different strains furthermore support that PHSP12-driven expression causes a metabolic burden. For both strains expressing a fluorescent protein under control of PHSP12, the non-growth associated glucose consumption rates were higher (0.018 ± 0.007 g/g/h for the secreting strain ScNTp002 and 0.017 ± 0.001 g/g/h for intracellular production strain ScNTi001) compared to both the parental strain CEN.PK113-7D (0.0071 ± 0.001 g/g/h [7]) and PTEF1-based secreting strain ScNTp001 (0.0079 ± 0.004 g/g/h). This non-growth associated glucose consumption includes the glucose required for maintenance, but also part of the glucose used for product formation. However, for strain ScNTp001 (PTEF1), this value was similar to the rate of the parental strain while this strain secreted more recombinant protein implying that the majority of the non-growth associated glucose consumption is used for maintaining cellular homeostasis. The increase in non-growth associated glucose consumption is hence likely caused by the (strong) activation of the PHSP12 and associated metabolic stress, regardless of copy number.
Overall, the PTEF1 yielded higher extracellular protein titers, with increasing protein secretion efficiency at very low growth rates. The PHSP12 in contrast resulted in enhanced intracellular protein production when growth slowed down. Reduced protein secretion efficiencies, high intracellular accumulation and negative impacts on cellular energetics strongly suggest that the multiple copies of the PHSP12-SPKSH1-ymNeongreen expression cassette caused a negative feedback loop causing cellular stress. To explore the full potential of the PHSP12 promoter for secreted recombinant protein production, this may therefore have to be implemented in strains with superior secretion capacities [46]. In addition, targeted integration of (multiple) copies of the expression cassette can increase control over the expression level and thereby improve strain stability and reduce the metabolic burden.
Conclusion
To improve biobased production processes and especially precision fermentation for food proteins, increased titers, rates and yields are needed. Uncoupling production from growth can improve product yields but requires that specific production rates (qP) are maintained at (near-) zero growth rates. Here we show that using two different strong promotors, recombinant protein production can be uncoupled from specific growth rate, without the addition of inducers. The PHSP12 resulted in increased product yields for intracellular proteins at near-zero growth rates and 15-fold higher intracellular protein titers compared to the benchmark PTEF1. PTEF1 on the other hand led to increased extracellular protein titers. PTEF1 furthermore resulted in increasing protein secretion (qPex) at lower specific growth rates (µ) and increased secretion efficiencies.
Even though expression and especially secretion efficiencies can be protein specific, our results, using fluorescent proteins as markers for functional protein production, highlight that distinct optimization strategies are required for intracellular versus extracellular recombinant protein production.
Supplementary Information
Acknowledgements
The authors would like to thank Fred van den End and Wendy Evers for their technical assistance.
Abbreviations
- cX
Biomass concentration
- mP
Specific growth-rate independent product formation rate
- mS
Specific growth-rate independent glucose uptake rate
- MX
Total biomass amount
- µ
Specific growth rate
- PTEF1
Translation elongation factor 1alpha promotor
- PHSP12
Heat shock protein 12 promoter
- qP
Specific product formation rate
- qPex
Specific product secretion rate
- qS
Specific glucose uptake rate
- rP
Volumetric product formation rate
- SPKsh1
Ksh1 signal peptide
- TEP
Extracellular protein titer
- TIP
Intracellular protein titer
- YXS
Yield of biomass on glucose
- YPS
Yield of product on glucose
Author contributions
NT: Investigation, writing and revising original draft, reviewing and editing, methodology, conceptualization, formal analysis. WHB: Investigation, methodology. RAW: conceptualisation, reviewing and editing. MMMB: conceptualization, formal analysis, supervision, writing and revising original draft, reviewing, editing, methodology.
Funding
The research performed for this study has in part been funded by a stipend from Ministry of National Education of the Republic of Turkey to N. Temelli.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nielsen MB, Meyer AS, Arnau J. The next food revolution is here: Recombinant microbial production of milk and egg proteins by precision fermentation. Annual Rev Food Sci Technol. 2024;15:173–87. [DOI] [PubMed] [Google Scholar]
- 2.Boender LGM, De Hulster EAF, Van Maris AJA, Daran-Lapujade PAS, Pronk JT. Quantitative physiology of Saccharomyces cerevisiae at near-zero specific growth rates. Appl Environ Microbiol. 2009;75:5607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pirt SJ. Maintenance energy: a general model for energy-limited and energy-sufficient growth. Arch Microbiol. 1982;133:300–2. [DOI] [PubMed] [Google Scholar]
- 4.Vos T, de la Torre Cortés P, van Gulik WM, Pronk JT, Daran-Lapujade P. Growth-rate dependency of de Novo Resveratrol production in Chemostat cultures of an engineered Saccharomyces cerevisiae strain. Microb Cell Fact. 2015;14:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ercan O, Bisschops MMM, Overkamp W, Jørgensen TR, Ram AF, Smid EJ, et al. Physiological and transcriptional responses of different industrial microbes at near-zero specific growth rates. Appl Environ Microbiol. 2015;81:5662–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rebnegger C, Coltman BL, Kowarz V, Peña DA, Mentler A, Troyer C et al. Protein production dynamics and physiological adaptation of Recombinant Komagataella phaffii at near-zero growth rates. Microb Cell Fact. 2024;23. [DOI] [PMC free article] [PubMed]
- 7.Temelli N, van den Akker S, Weusthuis RA, Bisschops MMM. Exploring yeast’s energy dynamics: the general stress response lowers maintenance energy requirement. Microb Biotechnol. 2025;18. [DOI] [PMC free article] [PubMed]
- 8.Vos T, Hakkaart XDV, Hulster EAF, Maris AJA, Pronk JT, Daran-Lapujade P. Maintenance-energy requirements and robustness of Saccharomyces cerevisiae at aerobic near-zero specific growth rates. Microb Cell Fact. 2016;15:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bisschops MMM, Luttik MAH, Doerr A, Verheijen PJT, Bruggeman F, Pronk JT, et al. Extreme calorie restriction in yeast retentostats induces uniform non-quiescent growth arrest. Biochim Biophys Acta Mol Cell Res. 2017;1864:231–42. [DOI] [PubMed] [Google Scholar]
- 10.Rebnegger C, Vos T, Graf AB, Valli M, Pronk JT, Daran-Lapujade P, et al. Pichia pastoris exhibits high viability and a low maintenance energy requirement at near-zero specific growth rates. Appl Environ Microbiol. 2016;82:4570–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, et al. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell. 2000;11:4241–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Alessio Y, D’Alfonso A, Camilloni G. Chromatin conformations of HSP12 during transcriptional activation in the Saccharomyces cerevisiae stationary phase. Adv Biol Regul. 2023;90. [DOI] [PubMed]
- 13.Bai C, Tesker M, Melamed-Kadosh D, Engelberg D, Admon A. Hog1-induced transcription of RTC3 and HSP12 is robust and occurs in cells lacking Msn2, Msn4, Hot1 and Sko1. PLoS ONE. 2020;15:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernat-Camps N, Ebner K, Schusterbauer V, Fischer JE, Nieto-Taype MA, Valero F et al. Enabling growth-decoupled Komagataella phaffii Recombinant protein production based on the methanol-free PDH promoter. Front Bioeng Biotechnol. 2023;11. [DOI] [PMC free article] [PubMed]
- 15.Decoene T, De Maeseneire SL, De Mey M. Modulating transcription through development of semi-synthetic yeast core promoters. PLoS ONE. 2019;14. [DOI] [PMC free article] [PubMed]
- 16.David F, Nielsen J, Siewers V. Flux control at the malonyl-CoA node through hierarchical dynamic pathway regulation in Saccharomyces cerevisiae. ACS Synth Biol. 2016;5:224–33. [DOI] [PubMed] [Google Scholar]
- 17.Partow S, Siewers V, Bjørn S, Nielsen J, Maury J. Characterization of different promoters for designing a new expression vector in Saccharomyces cerevisiae. Yeast. 2010;27:955–64. [DOI] [PubMed] [Google Scholar]
- 18.Hou J, Tyo KEJ, Liu Z, Petranovic D, Nielsen J. Metabolic engineering of Recombinant protein secretion by Saccharomyces cerevisiae. FEMS Yeast Res. 2012;12:491–510. [DOI] [PubMed] [Google Scholar]
- 19.Rebnegger C, Graf AB, Valli M, Steiger MG, Gasser B, Maurer M, et al. In Pichia pastoris, growth rate regulates protein synthesis and secretion, mating and stress response. Biotechnol J. 2014;9:511–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nijkamp JF, van den Broek M, Datema E, de Kok S, Bosman L, Luttik MA et al. De Novo sequencing, assembly and analysis of the genome of the laboratory strain Saccharomyces cerevisiae CEN.PK113-7D, a model for modern industrial biotechnology. Microb Cell Fact. 2012;11. [DOI] [PMC free article] [PubMed]
- 21.Mikkelsen MD, Buron LD, Salomonsen B, Olsen CE, Hansen BG, Mortensen UH, et al. Microbial production of indolylglucosinolate through engineering of a multi-gene pathway in a versatile yeast expression platform. Metab Eng. 2012;14:104–11. [DOI] [PubMed] [Google Scholar]
- 22.Lee ME, DeLoache WC, Cervantes B, Dueber JE. A highly characterized yeast toolkit for modular, multipart assembly. ACS Synth Biol. 2015;4:975–86. [DOI] [PubMed] [Google Scholar]
- 23.Botman D, de Groot DH, Schmidt P, Goedhart J, Teusink B. In vivo characterisation of fluorescent proteins in budding yeast. Sci Rep. 2019;9. [DOI] [PMC free article] [PubMed]
- 24.O’Riordan NM, Jurić V, O’Neill SK, Roche AP, Young PW. A yeast modular cloning (MoClo) toolkit expansion for optimization of heterologous protein secretion and surface display in Saccharomyces cerevisiae. ACS Synth Biol. 2024;13:1246–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Juergens H, Varela JA, de Vries ARG, Perli T, Gast VJM, Gyurchev NY, et al. Genome editing in Kluyveromyces and Ogataea yeasts using a broad-host-range Cas9/gRNA co-expression plasmid. FEMS Yeast Res. 2018;18:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mans R, van Rossum HM, Wijsman M, Backx A, Kuijpers NGA, van den Broek M, et al. CRISPR/Cas9: A molecular Swiss army knife for simultaneous introduction of multiple genetic modifications in Saccharomyces cerevisiae. FEMS Yeast Res. 2015;15:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gietz RD, Schiestl RH. High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat Protoc. 2007;2:31–4. [DOI] [PubMed] [Google Scholar]
- 28.Verduyn C, Postma E, Scheffers WA, Van Dijken JP. Physiology of Saccharomyces cerevisiae in anaerobic glucose-limited Chemostat cultures. J Gen Microbiol. 1990;136:395–403. [DOI] [PubMed] [Google Scholar]
- 29.Entian KD, Schuster T, Hegemann JH, Becher D, Feldmann H, Güldener U, et al. Functional analysis of 150 deletion mutants in Saccharomyces cerevisiae by a systematic approach. Mol Gen Genet. 1999;262(4–5):683–702. [DOI] [PubMed] [Google Scholar]
- 30.Postma E, Verduyn C, Scheffers WA, Van Dijken JP. Enzymic analysis of the Crabtree effect in glucose-limited Chemostat cultures of Saccharomyces cerevisiae. Appl Environ Microbiol. 1989;55:468–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boender LGM, Almering MJH, Dijk M, van Maris AJA, de Winde JH, Pronk JT, et al. Extreme calorie restriction and energy source starvation in Saccharomyces cerevisiae represent distinct physiological States. Biochim Biophys Acta Mol Cell Res. 2011;1813:2133–44. [DOI] [PubMed] [Google Scholar]
- 32.Ferndahl C, Bonander N, Logez C, Wagner R, Gustafsson L, Larsson C, et al. Increasing cell biomass in Saccharomyces cerevisiae increases Recombinant protein yield: the use of a respiratory strain as a microbial cell factory. Microb Cell Fact. 2010;9:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zahrl RJ, Prielhofer R, Burgard J, Mattanovich D, Gasser B. Synthetic activation of yeast stress response improves secretion of Recombinant proteins. N Biotechnol. 2023;73:19–28. [DOI] [PubMed] [Google Scholar]
- 34.Holec PV, Camacho KV, Breuckman KC, Mou J, Birnbaum ME. Proteome-Scale screening to identify High-Expression signal peptides with minimal N-Terminus biases via yeast display. ACS Synth Biol. 2022;11:2405–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tyo KEJ, Liu Z, Magnusson Y, Petranovic D, Nielsen J (2014) Impact of protein uptake and degradation on recombinant protein secretion in yeast. Appl Microbiol Biotechnol 98:7149–7159 [DOI] [PubMed]
- 36.Wang Y, Li X, Chen X, Nielsen J, Petranovic D, Siewers V (2021) Expression of antibody fragments in Saccharomyces cerevisiae strains evolved for enhanced protein secretion. Microb Cell Fact 20(1):134 [DOI] [PMC free article] [PubMed]
- 37.Alper H, Fischer C, Nevoigt E, Stephanopoulos G. Tuning genetic control through promoter engineering. Proc Natl Acad Sci U S A. 2005;102(36):12678–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vogl T, Glieder A. Regulation of Pichia pastoris promoters and its consequences for protein production. N Biotechnol. 2013. 2013;30(4):385–404. [DOI] [PubMed]
- 39.Adnan M, Ma X, Olsson S, Wang J, Liu G. Promoter regulation and genetic engineering strategies for enhanced cellulase expression in Trichoderma Reesei. Microbiol Res. 2022;259:127011. [DOI] [PubMed] [Google Scholar]
- 40.Peng B, Williams TC, Henry M, Nielsen LK, Vickers CE. Controlling heterologous gene expression in yeast cell factories on different carbon substrates and across the Diauxic shift: A comparison of yeast promoter activities. Microb Cell Fact. 2015;14:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xia J, Sánchez BJ, Chen Y, Campbell K, Kasvandik S, Nielsen J. Proteome allocations change linearly with the specific growth rate of Saccharomyces cerevisiae under glucose limitation. Nat Commun. 2022;13:2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Regenberg B, Grotkjær T, Winther O, Fausbøll A, Åkesson M, Bro C, et al. Growth-rate regulated genes have profound impact on interpretation of transcriptome profiling in Saccharomyces cerevisiae. Genome Biol. 2006;7(11):R107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stadlmayr G, Mecklenbräuker A, Rothmüller M, Maurer M, Sauer M, Mattanovich D, et al. Identification and characterisation of novel Pichia pastoris promoters for heterologous protein production. J Biotechnol. 2010;150:519–29. [DOI] [PubMed] [Google Scholar]
- 44.Johansson N, Quehl P, Norbeck J, Larsson C. Identification of factors for improved ethylene production via the ethylene forming enzyme in Chemostat cultures of Saccharomyces cerevisiae. Microb Cell Fact. 2013;12:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boer VM, Crutchfield CA, Bradley PH, Botstein D, Rabinowitz JD. Growth-limiting intracellular metabolites in yeast growing under diverse nutrient limitations. Mol Biol Cell. 2010;21(1):198–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sosa-Carrillo S, Galez H, Napolitano S, Bertaux F, Batt G. Maximizing protein production by keeping cells at optimal secretory stress levels using real-time control approaches. Nat Commun. 2023;14. [DOI] [PMC free article] [PubMed]
- 47.Liu Z, Hou J, Martínez JL, Petranovic D, Nielsen J. Correlation of cell growth and heterologous protein production by Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2013;97:8955–62. [DOI] [PubMed] [Google Scholar]
- 48.Sidrauski C, Walter P. The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell. 1997;90(6):1031–9. [DOI] [PubMed] [Google Scholar]
- 49.Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 2000;101(3):249–58. [DOI] [PubMed] [Google Scholar]
- 50.Chen X, Li X, Ji B, Wang Y, Ishchuk OP, Vorontsov E, et al. Suppressors of amyloid-β toxicity improve Recombinant protein production in yeast by reducing oxidative stress and tuning cellular metabolism. Metab Eng. 2022;72:311–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.






