Sulfuric Acid
Sulfuric Acid
Customer Solutions
Electrochemical sulfuric acid production will become the standard for next generation cleaner sulfuric acid supply. The sulfuric acid product is tailored for critical mineral digestion and hydrometallurgical processing application requirements.
Travertine Products
Sulfuric Acid

Travertine Products
Purified Phosphoric Acid

Travertine Products
Cementitious Materials

Travertine Products
Carbon Dioxide Removal Credits
Travertine Products
Extracting More Value

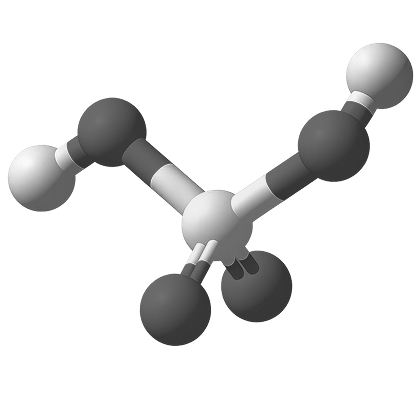
The most widely produced and used industrial chemical globally
-
Market Value
+$15B
Sulfuric Acid
-
Growth
+20%
Sulfuric Acid Growth by 2030
-
Volume
+260 M
MT / Sulfuric acid per year

Benefits

Existing SA production depends on burning sulfur from Oil & Gas to make sulfuric acid. Travertine SA production recycles sulfur within a closed loop, minimizing any sulfur feedstock requirement.

Existing SA production requires large plants to achieve economics of scale. Travertine SA production uses a modular electrochemical units that allow smaller scale plants.

Most SA ends up as gypsum waste after use. Travertine recycles the sulfur and makes cementitious materials, gypsum-free.

Applications
In copper heap leaching, sulfuric acid acts as a powerful lixiviant that chemicaly dissolves copper from oxide and some sulfide ores. This process begins by spraying a dilute sulfuric acid solution over large heaps of crushed ore. As the acid percolates through the pile, it reacts with copper minerals to form a water-soluble copper sulfate solution, known as the pregnant leach solution (PLS). The PLS is then collected at the base of the heap for downstream solvent extraction and electrowinning (SX-EW), where pure copper cathodes are produced.
Application Highlights
-
Lithium

Sulfuric Acid is used to extract lithium from spodumene ore.
-
Rare Earths

Sulfuric Acid is used to extract rare earths from REE rich ore.
-
Nickel

Sulfuric Acid is used to extract nickel from laterite ores.
-
Copper
Sulfuric Acid is used to extract copper from copper oxide and sulfide ores.
